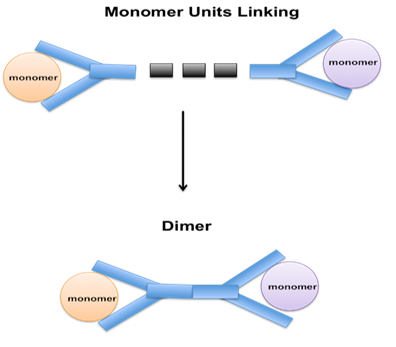
A dimerization process is a form of polymerization that converts two propylene molecules to generate one C6 olefin molecule that is termed dimate, which is then used as a gasoline mix stock. Propylene and butylene can be processed through a polymerization unit to create polymer gasoline, which is a blend stock for making gasoline.
In petroleum refining, dimerization is used to increase the octane rating of gasoline. The process involves the reaction of two smaller molecules, typically C4 to C6 hydrocarbons, under the presence of a catalyst to form a single molecule of higher molecular weight, usually C8 to C12. This dimer product can be blended with other gasoline components to produce a high-octane fuel.
Since alkylation units may use isobutane in addition to an olefin (increasing the final volume of gasoline) and produce a higher quality product, they have virtually supplanted polymerization. But Dimerization is a form of polymerization that uses only propylene as its olefin feed.
Dimerization Process
Dimerization is a chemical process that involves the combination of two identical molecules to form a single molecule with twice the molecular weight. The process is used in various industries, including petroleum refining, to create larger molecules with desirable properties.
The dimerization reaction is usually carried out under high temperature and pressure conditions, typically between 150°C to 300°C and 500 to 2000 psi. The choice of catalyst depends on the specific reactants used and the desired product, but commonly used catalysts include aluminium chloride, boron trifluoride, and zeolites.
The dimerization process can be carried out in batch or continuous mode. In batch mode, the reactants and catalyst are added to a reactor vessel, and the reaction is allowed to proceed until the desired conversion is achieved. The product is then separated from the catalyst and other by-products.
In continuous mode, the reactants and catalyst are continuously fed into a reactor vessel, and the product is continuously removed, with the unreacted material and catalyst being recycled. This allows for higher throughput and more efficient use of resources.
Dimerization Reaction Example
An example of a dimerization reaction is the conversion of two molecules of propylene (C3H6) to form a single molecule of hexene (C6H12) using a catalyst such as aluminium chloride or boron trifluoride. The reaction can be written as:
2C3H6 (propylene) –> C6H12 (hexene)
The reaction is typically carried out at high temperature and pressure in the presence of the catalyst. The resulting hexene product can be used as a feedstock for the production of various chemicals, such as plastics, detergents, and synthetic lubricants. In petroleum refining, dimerization of butene and pentene can be used to produce a high-octane gasoline component called iso-octene.
Another example of Dimerization is a process used in refineries to convert a lower molecular weight olefin (such as propylene or butylene) into a higher molecular weight olefin (such as octene or decene) by combining two identical molecules. This process is also known as oligomerization or polymerization.
For example, propylene dimerization involves the combination of two propylene molecules to form a single molecule of butene. This reaction is typically catalyzed by an acid catalyst, such as phosphoric acid or zeolite.
The resulting butene molecule can then undergo further dimerization or oligomerization to form higher molecular weight olefins, such as octene or decene. These higher molecular weight olefins are important feedstocks for the production of a variety of chemical products, including plastics, synthetic rubbers, and lubricants.
References
- Dimerization unit | McKinsey Energy Insights.
- Polymerization unit | McKinsey Energy Insights.
- Handbook of petroleum refining processes by R. Meyers
- B. Mostajeran et al., “Production of isooctane from isobutene: energy integration and carbon dioxide abatement via catalytic distillation,” ACS Publications