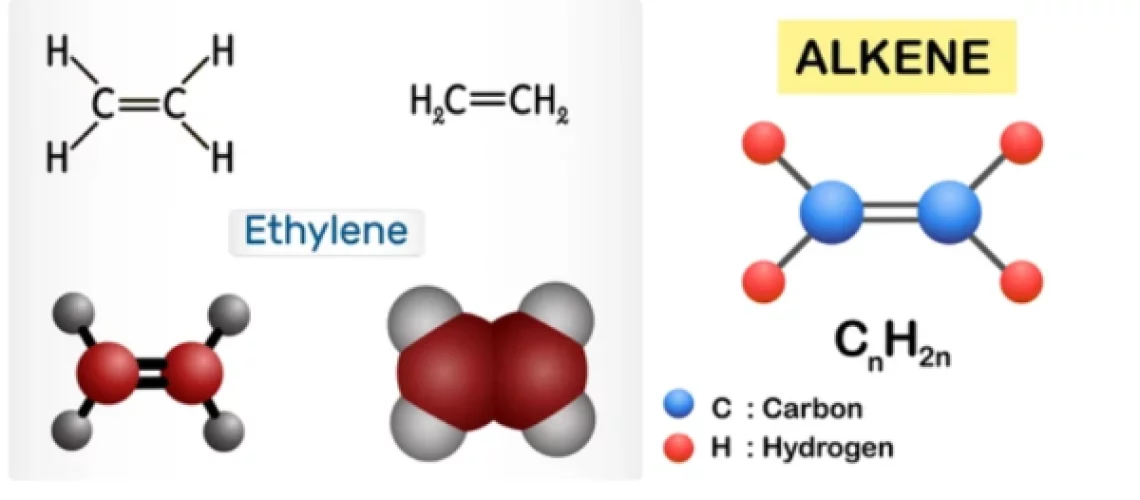
Olefins, also called alkenes or alkynes are compounds made up of hydrogen and carbon that contains one or more pairs of carbon atoms linked by double or triple bonds. Olefins do not occur naturally but are produced in large refining and petrochemical conversion processes. Olefins are examples of unsaturated hydrocarbons, are an important feedstock for the petrochemical industry, and are also very valuable components in the petroleum industry.
Olefins are classified as cyclic or acyclic (aliphatic) olefins, in which the double bond is located between carbon atoms forming part of a cyclic (closed-ring) or of an open-chain grouping, respectively, and as mono olefins, diolefins, triolefins, etc., in which the number of double bonds per molecule is, respectively, one, two, three, or some other number. Olefins containing carbon double and triple bonds are also called alkenes and alkynes respectively. Olefins have a general formula of CnH2n.
Olefins are more reactive than alkanes due to the presence of the double bond, which can be easily broken to form new bonds. The double bond in olefins is more reactive than the single b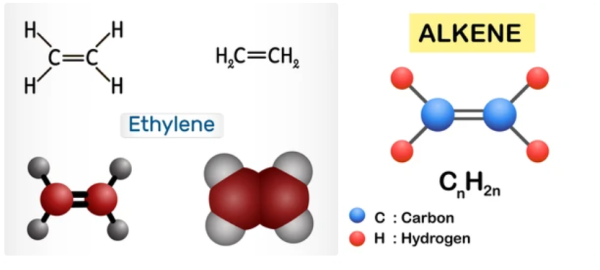
Production of Olefins
In petroleum refineries, Olefins are produced in thermal cracking units like Visbreaking, Coking, and Delayed coking. The fluid catalytic cracking unit produces Olefins mainly in the range of light hydrocarbon range C3–C5, which are used as petrochemical feedstocks and for alkylate production. Deep catalytic cracking (DCC) is a catalytic cracking process that selectively cracks a wide variety of feedstocks into light olefins and produces more Olefins as compared to FCC. Separation of these olefins from catalytic and thermal cracking gas streams could be achieved using physical and chemical separation methods.
In the petrochemical industry which requires Olefins in bulk quantity, most of the olefins are produced by steam cracking of hydrocarbon. The feedstocks for steam cracking units range from light paraffinic hydrocarbon gases to various petroleum fractions and residues. These as light olefins can also be produced by pyrolysis and fluid catalytic cracking of the vacuum distillates.
Impacts of Olefins on Petroleum Refinery Operations
Some major roles of Olefins in petroleum refineries are as follows;
- The presence of Olefins in gasoline and diesel range intermediate products makes them unstable and polymerizes them during storage. If the polymers are formed then they can clog and foul feed filters, and feed exchangers of hydrotreating, isomerization, alkylation, and other processing units.
- As olefins are highly reactive, exotherms are produced and can polymerize at the catalyst bed as well during saturation, therefore, special moderate active guard bed catalysts are applied in hydrotreating reactors. Further, as olefins produce exotherms on reactor beds, required temperatures are achieved through the saturation of Olefins. Otherwise, to achieve the required temperature on the reactor, most energy is required in the Feed heater.
- The presence of Olefins in petroleum products like diesel and gasoline, olefins contributes to octane, which is beneficial. However, because they tend to form harmful deposits inside engines, there are typically limits set on total olefin content.
- In a refinery, olefins are an important feedstock for the alkylation unit to produce alkylate (a high-value gasoline blend stock). The olefins most commonly used in the alkylation unit are butylene and propylene, which primarily come from the FCC unit.
- Naphtha feed before use as feed for a catalytic reforming unit is hydrotreated to saturate the olefins. Olefinic compounds are undesirable because they are precursors for coke, which deactivates the catalyst.
Olefins in Petrochemical Industry
Olefins such as ethylene (CH2=CH2) and propylene (CH3CH=CH2), are important sources of industrial chemicals and plastics; the diolefin derivative butadiene (CH2=CHCH=CH2) is used in making synthetic rubber. Olefins are the basis for polymers and oligomers used in plastics, resins, fibers, elastomers, lubricants, and gels. These as light olefins are industrially produced by pyrolysis and fluid catalytic cracking of the vacuum distillates. Another potential technique for light olefins production is the direct conversion of syngas.
Properties of Olefins
1. Physical Properties
Olefins are typically gases or liquids at room temperature, with lower molecular weight olefins existing as gases and higher molecular weight olefins existing as liquids. The boiling point of olefins is lower than that of their corresponding alkanes due to the presence of the double bond, which makes them more volatile. The density of olefins is also lower than that of their corresponding alkanes, which makes them useful in various applications such as in the production of polymeric materials.
2. Reactivity
Olefins are more reactive than alkanes due to the presence of the double bond, which can be easily broken to form new bonds. The double bond in olefins is more reactive than the single bond in alkanes because it contains a higher electron density. This makes olefins susceptible to various types of reactions such as addition, oxidation, and polymerization.
3. Isomerization
Olefins can exist as different isomers, which are compounds with the same molecular formula but different structures. This property is due to the possibility of having different positions of the double bond in the hydrocarbon chain. The position of the double bond can affect the physical and chemical properties of the compound, including its boiling point, reactivity, and stability.
4. Polymerization
One of the most important properties of olefins is their ability to undergo polymerization, which is the process of linking monomers together to form a polymer. This process is initiated by the reaction of the double bond in the olefin with a catalyst. The resulting polymer can have various physical and chemical properties, depending on the monomer used and the conditions of the reaction. Some examples of polyolefins include polyethylene, polypropylene, and polystyrene, which are widely used in the production of various products such as packaging materials, toys, and household appliances.
5. Solubility
Olefins are generally insoluble in water due to their nonpolar nature, but they are soluble in organic solvents such as ethanol and chloroform. This property makes olefins useful in various applications such as in the production of adhesives and coatings.
Top References
- www.mckinseyenergyinsights.com
- Alkenes (Inetchopen) Edited by Reza Davarnejad
- Chemistry of Petrochemical Processes Second Edition by Sami Matar and Lewis F. Hatch
- www.britannica.com