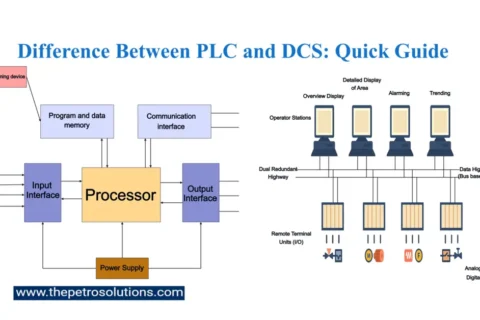
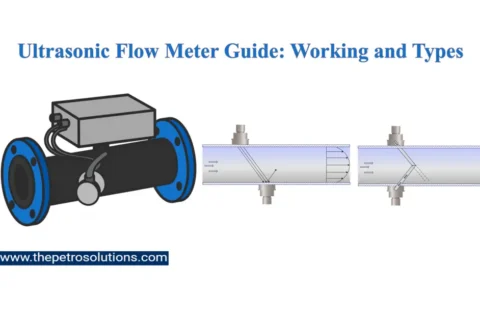
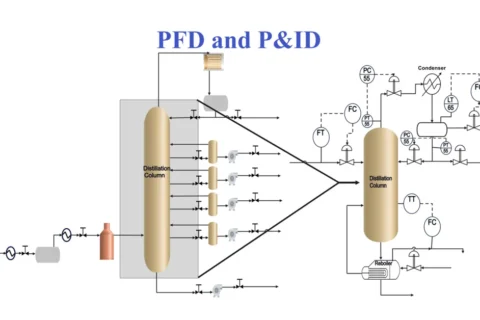
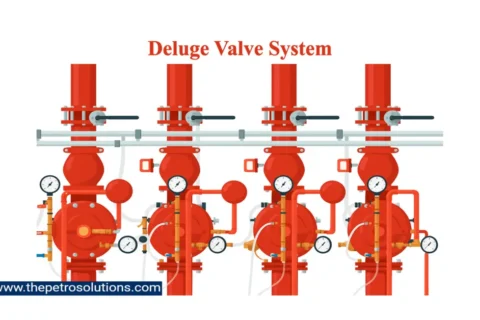
Kerosene, also called paraffin or paraffin oil, is a flammable pale-yellow or colourless petroleum product with a characteristic odour, intermediate in volatility between gasoline and diesel oil. It is produced by fractional distillation of crude oil and further treated in downstream units to improve product quality. It can also be manufactured from coal, Oil Shale, and Wood, naturally occurring substances. The primary characteristics of Kerosene are flash point, distillation range, burning characteristics, sulfur content, colour, and cloud point.
Uses of Kerosene
Kerosene was one of the most significant refinery products and was commonly used in oil lamps until electric lighting became popular. Kerosene is widely used today as an aviation fuel, heating oil, illumination, solvent, and a cutter stock in refinery fuel oils, etc. Significant amounts of kerosene are used as solvents for greases and insecticides. Further, treated kerosene is also used as a blending feedstock in the diesel fuel pool.
Kerosene Production in Petroleum Refinery
Kerosene or kerosine is mainly produced by fractional distillation of crude oil in a petroleum oil refinery. In addition, to crude oil distillation, kerosene is also produced in hydrocracking or catalytic cracking unit by cracking heavy hydrocarbons.
Straight-run kerosene is treated through a hydrotreating process and is applied to remove impurities like sulfur, nitrogen, and aromatics saturation required to meet the strict smoke point specifications associated with this finished product. Further, The MEROX process for kerosene/jet fuel oil sweetening is a process applied to control the mercaptans using air and caustic soda (NaOH) and is called Kero MEROX Process.
The ASTM International standard specification D-3699-19 defines two grades of kerosene: grades 1-K (less than 0.04% sulfur by weight) and 2-K (0.3% sulfur by weight). 1-K-grade kerosene is superior and burns cleaner with lower deposits, fewer toxins, and less frequent maintenance than 2-K-grade kerosene. Therefore, the 1-K grade is the preferred grade of kerosene for indoor kerosene heaters and stoves.
Properties of Kerosene
Some of the major physical and chemical properties of kerosene are as follows;
1. Density
Kerosene is manufactured from hydrocarbons obtained from the fractional distillation of petroleum at temperatures ranging from 150 to 280 °C, producing a mixture with a density of 0.78-0.81 g/cm3.
2. Nature of Kerosene
It is miscible in petroleum solvents but not soluble in water, showing that it is non-polar. We know that the polar substance dissolves in a polar solvent and the non-polar substance dissolves in the non-polar solvent by the like-like phenomena.
3. Molecular Formula
Kerosene comprises hydrocarbon molecules that typically have between 6 and 20 carbon atoms per molecule, with the majority having 9 to 18 carbon atoms. The molecular formula of Kerosene is C12H26−C18H38.
4. Composition of Kerosene
Irrespective of the crude oil source and its processing procedure, naphthenes (cycloalkanes) and branched- and straight-chain alkanes (hydrocarbon chains) make up at least 70% of the volume of Kerosene. Alkylbenzenes, which have a single ring, and alkyl naphthalenes, which have a double ring, are made from no more than 25% of the volume of the kerosene stream. Typically, olefins are comprised no more than 5% of the total volume.
5. Gross Calorific Value (GCV) and Net Calorific Value (NCV)
Kerosene has a similar heat of combustion to diesel fuel. Its lower heating value (NCV) is 43.1 MJ/kg (around 18,500 Btu/lb), and its higher heating value (GCV) is 46.2 MJ/kg (19,900 Btu/lb).
6. Flash Point
Kerosene is liquid at room temperature and has a minimum flash point of ~38 °C and its auto-ignition temperature is 220 °C (428 °F). Flash Point is necessary for functioning and safety purposes.
7. Acidity
Acids can be present in kerosene during refining due to the crude oil source. These trace acid quantities are undesirable because of the possibility of metal corrosion and impairment of the burning characteristics and other properties of the kerosene.
8. Freezing Point
The freezing point of kerosene depends on the grade, with commercial aviation fuel standardized at −47 °C (−53 °F). The freezing point is the temperature at which the hydrocarbon liquid solidifies at atmospheric pressure. It is one of the most important properties of kerosene and jet fuels due to the very low temperatures faced at high altitudes in jet planes. If jet fuel freezes, it cannot be pumped into the engine and seizes its engine’s functioning.
9. Cloud and Pour Point
Kerosene has a cloud point of -21.1°C and a pour point of -25.1°C.
10. Viscosity
The effective utilization of many petroleum fuels depends on their kinematic viscosity, which influences how well they flow through pipelines via injection nozzles and orifices and how well they operate at different burner temperatures. The dynamic viscosity of Kerosene is 1.92Ns/ and the kinematic viscosity of Kerosene is 2.39/s.
11. Smoke Point
The smoke point of Kerosene is 25 mm. A fuel with a high aromatic content can be hydrotreated to improve its smoke point. The smoke point is the maximum height of smokeless flame in mm that can be achieved with a calibrated wick-fed lamp, using a wick of woven solid circular cotton of ordinary quality.
12. Volatility
Kerosene has lower volatility than gasoline. Because of this property, kerosene is a relatively safe fuel to store and handle.
13. Colour
Kerosene is a flammable, colourless, or pale-yellow oily liquid with a characteristic odour between gasoline and gas/diesel oil in terms of volatility. Kerosene’s colour doesn’t matter significantly, but if a product is darker than usual, the composition of Kerosene is changed due to impurity.
14. Distillation Range
At the 10% recovery point, kerosene has a maximum distillation temperature of 170 °C and a final boiling point of 280 °C.
15. Mercaptan Sulphur
Removal of unwanted compounds, including hydrogen sulfide, mercaptan sulfur, and corrosive sulfur, is one of the goals of the refining treatment process. Both hydrogen sulfide and mercaptans have unpleasant smells and are corrosive.
For more details; on the harmful effects of kerosene, first aid measures, fire fighting, properties, handling and storage please consult the Safety Data Sheet of Kerosene.
Top References
- Springer Handbookoƒ Petroleum Technology Hsu Robinson (Editors)
- Handbook of petroleum product analysis by James G. Speight
- Kerosene topic www.wikipedia.com
For further information, discussion and queries please comment in the box below or contact us at admin@ or follow us on Facebook & LinkedIn.