The petrochemical industry is a branch of the chemical industry that produces a wide range of chemical products from petroleum and natural gas. These chemical products are the building blocks for many everyday items such as plastics, polymers, synthetic rubbers, synthetic fibers, fertilizers, etc. The petrochemical industry is a major contributor to the global economy, with petrochemical products being used in virtually every aspect of modern life.
Primary raw materials of petrochemical feedstock preparations are natural gas and petroleum refinery products. Natural gas is used by steam cracking to produce methane, ethane, and propane-butane. Oil refineries produce olefins and aromatics by fluid catalytic cracking of petroleum fractions. Aromatics and Benze are also produced in the catalytic naphtha reforming unit in petroleum refinery. Olefins and aromatics are the building blocks for a wide range of materials such as solvents, detergents, and adhesives.
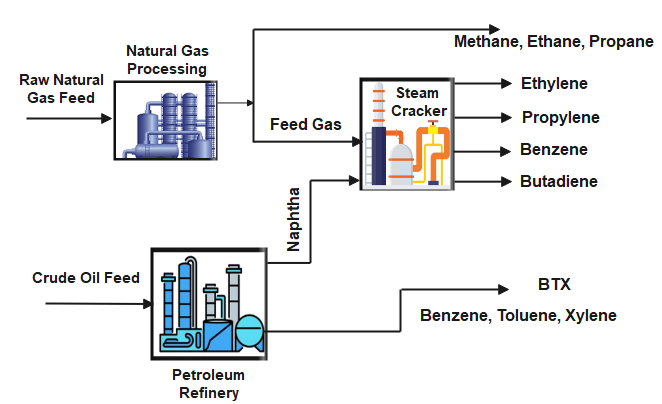
Petrochemical Intermediates
Petrochemical intermediates are the class of petrochemicals which are produced from the primary raw materials (i.e. Natural Gas and Petroleum) and will be further converted to finished products. These petrochemicals can also be called primary petrochemicals or basic petrochemicals and are divided into the following major groups depending on their chemical structure;
1. Paraffins
Paraffins such as methane (CH4), ethane (C2H6), propane (C3H8), the butane isomers (C4H10), and higher molecular weight hydrocarbon derivatives up to and including low-boiling mixtures such as naphtha.
2. Olefins
Olefins such as ethylene (CH2=CH2) and propylene (CH3CH=CH2), are important sources of industrial chemicals and plastics; the diolefin derivative butadiene (CH2=CHCH=CH2) is used in making synthetic rubber. Olefins are the basis for polymers and oligomers used in plastics, resins, fibers, elastomers, lubricants, and gels.
3. Aromatics
Aromatics such as benzene (C6H6), toluene (C6H5CH3), and the xylene isomers (CH3C6H4CH3) have a variety of uses;
- Benzene is a raw material for dyes, rubber, lubricants, drugs, explosives, pesticides and synthetic detergents. Further, benzene is used to produce (i) styrene, which is used in the manufacture of polymers and plastics; (ii) phenol, which is used in the manufacture of resins and adhesives by way of cumene; and (iii) cyclohexane, which is used in the manufacture of nylon.
- Toluene is used as a solvent for making paint, rubber, and adhesives, as a gasoline additive or for making toluene isocyanate which is used for making polyurethane foam), phenol, and trinitrotoluene known as TNT).
- Xylene is used as a solvent and as an additive for making fuels, rubber, leather and terephthalic acid which is used in the manufacture of polymers.
4. Synthesis Gas
Synthesis gas is a mixture of carbon monoxide (CO) and hydrogen (H2) that is sent to a Fischer–Tropsch reactor to produce naphtha-range and kerosene-range hydrocarbon derivatives as well as methanol (CH3OH) and dimethyl ether (CH3OCH3.
Petrochemical Finished Products
Using the above intermediates, a variety of plastics, rubber, fibers, solvent, paint, etc., are manufactured. Plastics are available in the form of extrudates, granules, powders, beads, etc. These are converted into plastic products, such as bags, films, furniture, and products of various shapes and sizes by casting, moulding, or blowing machines, as marketable products.
Synthetic fibers are made from polymers that have a high modulus of elasticity as compared to plastics and rubbers. These polymers are also available in the form of extrudates, powders, and beads, which are converted to fibers in a drawing mechanism and collected in bales.
Rubbers or elastomers are polymers with a low modulus of elasticity. Raw rubber is available from the polymerisation unit in the form of sheets, which are cut and blended with various chemical ingredients along with sulfur (known as vulcanization) to achieve the desired quality for making tyres and other products.
Chemical Reactions of Petrochemicals
Various chemical reactions are involved in the petrochemical manufacturing processes. Most of these reactions are catalytic with high-temperature effects. Examples of petrochemical manufacturing reactions are dissociation, dehydrogenation, hydrogenation, addition, polymerisation, and condensation.
Dissociation reactions are carried out during the thermal and catalytic cracking process. Dehydrogenation reactions are catalytic or thermal cracking types. Additional reactions, such as oxidation, chlorination, fluorination, and sulfonation of the parent olefin or aromatic hydrocarbons, are required to make intermediates or monomers. Polymerization reaction occurs mostly in the presence of catalysts with heat evolution.
Top References
- Handbook of Petrochemical Processes by James G. Speight
- Fundamentals of Petroleum and Petrochemical Engineering by Uttam Ray Chaudhri
- www.wkipedia.com





