Distillation is the process of separating two or more components from a liquid mixture by using selective boiling and condensation or their relative volatility inside the distillation tower. Distillation may result in essentially complete separation (resulting in nearly pure components), or it may be a partial separation that increases the concentration of selected components; in either case, the process makes use of differences in the relative volatility of the mixture’s components.
In industrial applications, distillation is a physical separation process. It is the most commonly applied separation technology worldwide in the process industry and is responsible for up to ~50% of both operating and capital costs. Further, distillation consumes ~ 50% of the total process energy used by the chemical and petroleum refining industries each year.
Working Principle of the distillation process
The basic principle behind the distillation process is that different liquids boil at different temperatures. So when a mixture is heated, the component with a lower boiling point or lower volatility starts to boil first and converts into vapours which can be then collected separately.
How Does The Distillation Process Work?
The feedstock enters the distillation column where column internals (trays, plates, or packing) provide vapour-liquid contact. When the mixture is heated to its boiling point, vaporization starts and the liquid changes to its gaseous phase. More about distillation column internals, please view the blog ” Types of Trays in Distillation Column“.
The vapour-rich stream with the more volatile or lighter component rises and exits at the top of the column and enters the overhead system of the distillation where condensation occurs. The overhead system can have fin fan exchangers or shell and tube heat exchangers where the vapours condense back to a liquid phase.
The condensed vapours accumulate in the reflux drum and exit as the distillate. Part of it is recycled back to the column as liquid reflux to control the tower top temperature. The higher the reflux flow, the lower will be the distillation tower’s top temperature, and as a result slow evaporation.
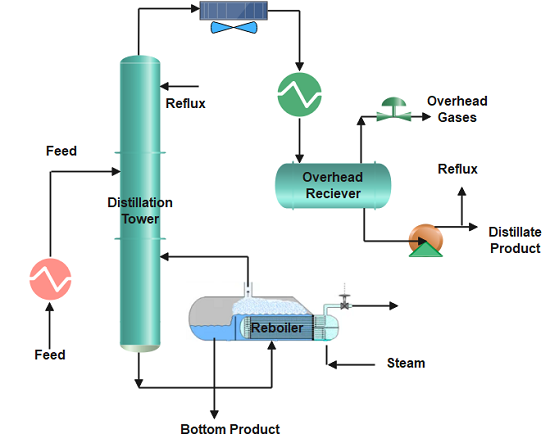
The liquid-rich stream exits at the bottom of the column and leaves the reboiler as the bottom’s product. The reboiler provides heat to the liquid at the bottom, and when it vaporizes, some of it is sent back to the column. Stripping steam is also frequently applied in place of reboilers. The bottom stream is then cooled by exchanging heat with the stripper feed.
Applications of Distillaiton
- Distillation is an effective and traditional method of desalination.
- Distillation is the first process in a petroleum refinery where crude oil is separated into various petroleum streams. In petroleum refineries, almost every unit has a distillation column in the form of a stripper, fractionator, or stabilizer. In the petroleum industry, oil stabilization is a form of partial distillation that reduces the vapour pressure of crude oil thereby making it safe for storage and transport as well as reducing the atmospheric emissions of volatile hydrocarbons. In midstream operations at oil refineries, fractional distillation is a major class of operation for transforming crude oil into fuels and chemical feedstocks.[2]
- The distillation of fermented products produces distilled beverages with high alcohol content or separates other fermentation products of commercial value.
- Cryogenic distillation leads to the separation of air into its components – notably oxygen, nitrogen, and argon – for industrial use.
- In the chemical industry, large amounts of crude liquid products of chemical synthesis are distilled to separate them, either from other products, from impurities, or from unreacted starting materials
Types of Distillation
Major types of Distillation process are as follows;
1. Simple Distillation
Simple distillation involves a liquid mixture of two components that are heated until the substance with a lower boiling point starts vaporizing. The vapours are then immediately condensed with the help of a condenser. Simple distillation is used to separate the mixture to have a significant boiling point difference of more than 25 degrees or when separating liquids from non-volatile solids or oils. The purity of the distillate (the purified liquid) is governed by Raoult’s law.
Raoult’s law Proposed by French chemist François-Marie Raoult in 1887, states that the partial pressure of each component of an ideal mixture of liquids is equal to the vapour pressure of the pure component (liquid or solid) multiplied by its mole fraction in the mixture. In consequence, the relative lowering of the vapour pressure of a dilute solution of nonvolatile solute is equal to the mole fraction of solute in the solution.
2. Vacuum Distillation
Vacuum distillation is distillation performed under reduced pressure, which allows the separation of compounds at low temperatures. It is applied for high boiling point compounds. This technique is used when the boiling point of the compound is difficult to achieve or will cause the compound to decompose. Vacuum distillation prevents the product degradation or polymerization of the compound due to operation at a lower temperature. Utilizing vacuum distillation can reduce the height and diameter, and thus the capital cost of a distillation column. In petroleum refinery, it is normally applied to convert reduced crude into gas oils, that as a result significantly improves the conversion of an oil refinery.
3. Fractional Distillation
Fractional distillation is the separation of a mixture into its component parts or fractions. Chemical compounds are separated by heating them to a temperature at which one or more fractions of the mixture will vaporize. Generally, the components or fractions have boiling points difference by less than 25 °C (45 °F) from each other under the pressure of one atmosphere. Fractional distillation is the most common form of separation technology used in petroleum refineries, petrochemical, and chemical plants, natural gas processing, and cryogenic air separation plants. In most cases, the distillation is operated at a continuous steady state.
4. Azeotropic distillation
Azeotropic distillation is the type of distillation in which another component is added to generate a new, lower-boiling compound called an azeotrope. The Azeotrope is heterogeneous (e.g. producing two or more, liquids phases), such as in the example below with the addition of benzene to water and ethanol.
This type of distillation is applied for components that cannot be separated by normal distillation due to nearly the same boiling points of the components. therefore an additional third component (known as an entrainer) is added to the mixture. This has the effect of changing the volatility of one of the liquids in the azeotrope to a greater extent than the other, allowing separation to occur.
5. Extractive Distillation
Extractive Distillation can be defined as distillation performed in the presence of a miscible, high-boiling, relatively non-volatile component, the solvent, that forms no azeotrope with the other components in the mixture. This method is used for mixtures having a low value of relative volatility, nearing unity. In this method, an additional component called entrainer is added to the mixture but it will not form any azeotrope but act differently with the component of the mixture and cause a change in relative volatility to allow the new three-part mixture to be separated by normal distillation
6. Steam Distillation
Steam distillation is the type of distillation in which steam is used as a stripping media instead of steam reboilers. Steam used reduced the vapour pressure of the boiling components thus separating them at low temperatures. Steam is condensed and separated from the stripper overhead system. In the petroleum and petrochemical industries, it is widely applied. Further, it costs less energy to perform separation as compared to steam reboilers to get the same separation although stripping steam cannot be recycled.
7. Batch Distillation
Batch distillation is the distillation that is performed in batches, meaning that a batch of the compound is distilled to separate it into its component fractions before the distillation still is again charged with more feedstock and the process is repeated. Batch distillation is applied for the production of seasonal, or low-capacity and high-purity chemicals. Batch distillation is frequently used in the pharmaceutical industry.
8. Continuous Distillation
Continuous distillation is a form of distillation in which the feedstock is continuously fed to a distillation column and is separated into fractions. The separated fractions are removed continuously as output streams. The lightest fraction is removed from the top while other fractions are removed as side cuts according to their boiling points. The bottom of the distillation column (or residuum) fraction, is the least volatile component of the feed mixture that has not been separately captured as a condensed vapour.
Top References
- www.wikipedia.com
- www.byjus.com
- www.toppr.com
- www.chemicaltweak.com
- www.theengineersperspectives.com
For further information, discussion and queries please comment in the box below or contact us at admin@ or follow us on Facebook & LinkedIn.





