Alkylation is a chemical process in a petroleum oil refinery in which a high-quality gasoline component (alkylate) is produced by the reaction of low-value light hydrocarbons of the LPG range i.e. olefins and paraffin. Isobutanes combine with Olefins (C3 ~C5) in the presence of strong acids to produce longer-branched chain hydrocarbons. Alkylate is a premium-quality gasoline blendstock with relatively high octane properties and no aromatic components. The alkylation process in petroleum refining is becoming popular as aromatics limits on gasoline have been tightened as per Euro specifications of gasoline fuel.
Product of Alkylation Process
The Alkylate which is the product of the alkylation unit is composed of a mixture of high-octane branched-chain paraffinic hydrocarbons (mostly isopentane and isooctane). Alkylate is a premium gasoline blending stock because it has extraordinary antiknock properties and clean burning. The octane number of the alkylate depends mainly upon the kind of alkenes used and the operating conditions of the Alkylation Unit. Alkylate hydrocarbon products have research octane numbers from 90 to 98 and are free from aromatics, benzene, and sulfur. These properties make the alkylate very suitable for petroleum refinery gasoline pools due to the implementation of Euro Specifications of Gasoline Fuel.
Feeds of Alkylation Process Unit
The olefin feedstock in a petroleum refinery to an alkylation unit generally comes from a Fluid Catalytic Cracking Unit (FCCU) and contains butene, isobutene, and possibly propane and amylenes. The isobutane feed to an alkylation unit can be either low or high purity. The low purity Isobutane feed (typically less than 70% vol isobutane) usually originates from the catalytic reforming unit in a refinery and is processed in the Deisobutanizer (DIB). High-purity feedstock ( greater than 95% vol isobutane) normally originates from an external Deisobutanizer (DIB) tower and is fed directly to the alkylation unit reaction zone.
Process Description of Alkylation Process Unit
Alkylation reactions are catalyzed by strong acids. If sulfuric acid is used, the unit is called the sulfuric acid alkylation unit (SAAU), and if hydrofluoric acid is used, the unit is called the hydrofluoric acid alkylation unit (HFAU). Both the Hydrofluoric acid Alkylation Unit (HFAU) and Sulfuric acid Alkylation Unit (SAAU) require expensive corrosion-resistant equipment. Safety in handling and operations is a major concern for both, which also face disposal issues associated with spent acids and acid-soluble oils. Please also view the blog “Hydrofluoric Acid Alkylation Unit Safety Measures”.
The general Process Description of the Alkylation Unit is as follows;
- Prior to entering the reaction section, the olefin and isobutane feeds are treated in a coalescer to remove water, sulfur, and other contaminants.
- A mixture of light olefins and isobutane (either C4s, C3s, or a mix) is injected into a reactor with a series of reaction chambers. These reaction chambers are filled with an acid catalyst; either sulfuric acid (H2SO4) or hydrofluoric acid (HF). Due to the presence of a catalyst, isobutane reacts with an olefin to produce either a C7 or C8 naphtha-range product called alkylate.
- The reactor effluent goes to a settler, in which hydrocarbons separate from the acid. The acid is separated and returned to the reactor. The hydrocarbons are washed with caustic and sent to a fractionator.
- The fractionation section comprises a depropanizer, a deisobutanizer, and a debutanizer. Alkylate from the deisobutanizer can go directly to motor-fuel blending, or it can be reprocessed to produce aviation-grade gasoline.
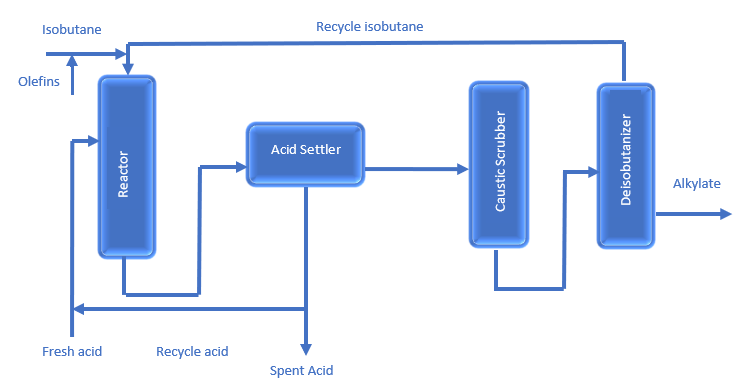
HF Alkylation Process Flow Diagram is given below;

Operating variables of the Alkylation Unit
Many operating variables impact the alkylate quality and operating costs of an alkylation unit whether it is an HFAU ( Hydrofluoric acid Alkylation Unit) or SAAU (Sulfuric Acid Alkylation unit).
a. Isobutane Concentration
In order to achieve the desired alkylation reactions, it is essential to maintain a high concentration of isobutane in the alkylation unit reaction zone. Low isobutane-olefin ratios increase the probability of olefin polymerization which results in lower octane. Polymerization reactions also cause a higher rate of production of acid-soluble oils, which results in higher acid consumption.
b. Temperature
Typically, alkylation is carried out below ~ 50 °C. Higher reaction temperatures dramatically proceed with the polymerization reactions that will dilute the acid. Equipment and piping corrosion will also increase with higher reaction temperatures. On the other side, low reaction temperatures slow the settling rate of the acid from the alkylate. Seasonal factors also affect the polymerization reactions, therefore in summer, the consumption of acid is higher, especially in HFAU (Hydrofluoric acid Alkylation Unit).
c. Acid strength
As the concentration of the acid catalyst is decreased, the production rate of acid-soluble polymers increases. High acid strength must be maintained in order to minimize polymerization and red oil production. When acid concentrations are too low then catalyst activity is considerably decreased and polymerization increases to the point that it is difficult to maintain acid strength. This condition is known as an acid runaway.
d. Olefin Space Velocity
Olefin space velocity is defined as the volume of olefin charged per hour divided by the average volume of sulfuric acid in the contactor reactor. Generally, higher olefin space velocities tend to rise the sulfuric acid consumption rates and decrease alkylate octane.
e. Mixing
Mixing is an important parameter, especially in SAAU (Sulfuric Acid Alkylation Unit) because the alkylation reaction depends on the emulsion of the hydrocarbon into the sulfuric acid. This is an acid continuous emulsion and it is assumed that the reaction happens at the interface of acid and hydrocarbon. The better the emulsion, the finer the droplets, and the better the reaction.
Commercial Processes of Alkylation Unit
The major licensor of the Hydroflouric Acid Alkylation Unit (HFAU) is UOP Honeywell and Sulfuric Acid Alkylation Unit (SAAU) is STRATCO Alkylation Technology licensed by DuPont, followed by the EMRE technology owned by ExxonMobil.
References
- The refinery of the future by James G.speight
- Springer Handbook of Petroleum Technology Chang Samuel Hsu and Paul R. Robinson
- Fundamentals of petroleum refining by M.A Fahi, T.A. Al-Sahhaf, A.S Elkilani
- Alkylation unit by Wikipedia
For further information, discussion and queries please comment in the box below or contact us at admin@ or follow us on Facebook & LinkedIn.
Certified Functional Safety Professional (FSP, TÜV SÜD), Certified HAZOP & PHA Leader, LOPA Practitioner, and Specialist in SIL Verification & Functional Safety Lifecycle, with 18 years of professional experience in Plant Operations and Process Safety across Petroleum Refining and Fertilizer Complexes.
- Nasir Hussainhttps://thepetrosolutions.com/author/admin/
- Nasir Hussainhttps://thepetrosolutions.com/author/admin/
- Nasir Hussainhttps://thepetrosolutions.com/author/admin/
- Nasir Hussainhttps://thepetrosolutions.com/author/admin/






