Hydrotreating is the reaction of organic compounds in the presence of Hydrogen to remove sulfur, nitrogen, metals, halides, and oxygen from the petroleum fractions ranging from LPG to vacuum residue. The hydrotreating process also transforms unsaturated olefins and aromatics into corresponding saturated hydrocarbons. Hydrotreating reactions are carried out in a reactor in the presence of hydrotreating catalysts Ni (Nickel), Mo (Molybdenum), Co (Cobalt), or Tungsten catalysts at high temperatures (300~400 C) and high pressure 10~100 bar. The type and amount of impurities in a petroleum product can be varied depending upon the type of hydrocarbon mixture in the feedstock. In general, light feeds (e.g., naphtha) contain fewer types of impurities and require less severe reactions. On the other hand, heavy feeds (e.g., vacuum residue) contain more complex contaminants, therefore require more serve Hydrotreating reactions.
More about the hydrotreating process can be found in my previous blog ” Hydrotreating Process in Oil Refinery”.
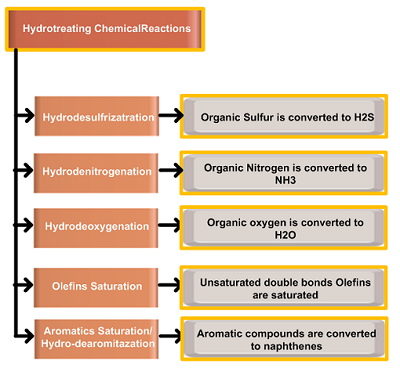
The main hydrotreating chemical reactions are Hydrodesulfrizatration (HDS) and Hydrodenitrogenation (HDN). All the hydrotreating reactions are exothermic, causing a rise in the reactor temperature as the feed passes through the hydrotreating catalyst bed. The reactor differential temperature (∆T) value depends on the concentration of each heteroatom and the extent of each reaction during hydrotreatment. The heat of the reaction varies significantly among the different reactions and from one compound to the other. As the hydrogen consumption increases to remove each with rising in organo compound, the amount of heat released also increases. The list of Hydrogenolysis reactions during the hydrotreating process is shown in the table below;
| Hydrotreating Reactions | Description of Reactions |
| Hydro-Desulfrizatration | Organic Sulfur is converted to H2S |
| Hydro-Denitrogenation | Organic Nitrogen is converted to NH3 |
| Hydro-Deoxygenation | Organic Oxygen is converted to H2O |
| Olefins Saturation | Unsaturated double bonds Olefins are saturated |
| Aromatics Saturation/ Hydro-dearomitazation |
Aromatic compounds are converted to naphthenes. |
| Hydro-Demetallation | Organometallics are converted to their respective metal sulfides |
| Hydro-Dehalogenation | Halides are converted to Halide Hydrogen compounds like HCL by reacting with Hydrogen. |
The behavior of hydrotreating reactions rates, heats of reaction, and hydrogen consumption is:
- Hydrodesulfurization and olefin hydrogenation is the most rapid reactions.
- Olefin saturation liberates the most heat per unit of hydrogen consumed.
- Hydrodenitrogenation and hydrodearomatization are the most difficult reactions.
- High Hydrogen consumption liberates the high heat of reaction.
Details of Hydrotreating reaction chemistry are described below;
1. Sulfur Removal or Hydrodesulfurization (HDS)
Sulfur removal is
also referred to as desulfurization or hydrodesulfurization (HDS) in which the organic sulfur compounds are converted to hydrogen sulfide. Sulfur is removed because of its environmental effects and poisonous nature for the downstream processes like Reforming and Isomerisation catalysts.
During the hydrodesulfurization process hydrogen breaks carbon‐sulfur bonds and saturates remaining hydrocarbon chains. There are six basic sulfur types present in feed i.e. mercaptans, sulfides, disulfides, thiophenes, benzothiophenes, and dibenzothiophenes. Removal of Sulfur from mercaptans, sulfides, disulfides is an easy process, while removal from the heteroatomic aromatic compounds is more difficult because it proceeds by ring-opening followed by Sulfur removal and saturation of the olefin group.
The relative ease of sulfur removal from a particular hydrocarbon feed depends greatly upon the type of Sulfur compound. In general, heavy hydrocarbon fractions contain more difficult species as compare to lighter hydrocarbon feeds.
Easiest to remove ⇒ the hardest to remove
Mercaptans ⇒ sulfides ⇒ disulfides ⇒ thiophenes ⇒ benzothiophenes ⇒ dibenzothiophenes
Hydrodulfrization reactions are as follows;
a. Mercaptanes: ![]()
b. Sulfides: ![]()
c. Di Sulfides: ![]()
d. Thiophenes:
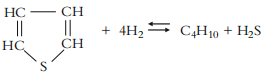
2. Nitrogen Removal or Hydrodenitrogenation (HDN)
Nitrogen removal is also referred to as denitrogenation or hydrodenitrogenation (HDN) in which the organic nitrogen compounds are converted to ammonia (NH3). Ammonia reacts with Chlorides and H2S to form Ammonium Sulfide and Chloride salts which are removed by dissolving in wash water in the form of sour water from a high-pressure separator boot. Nitrogen is an environmental pollutant and poisonous for the downstream catalysts of platforming and isomerization units.
Nitrogen is mostly found in the heavier petroleum fractions in the form of five and six-membered aromatic ring structures. The molecular complexity and quantity of nitrogen-containing molecules increase with the rise in boiling range and make them more difficult to convert. Denitrogenation reactions are more difficult than De-sulfurization reactions thus require more severe reaction conditions.
The hydrodenitrogenation reactions follow a different path as compared to the desulfurization reaction. In desulfurization, first, the sulfur is removed creating Olefin as an intermediate product which is then saturated while in denitrogenation, at first the aromatic ring is saturated and then the nitrogen is removed.
- The hydrodenitrogenation (HDN) reaction of Pyridine (C5H5N), a nitrogen compound occurs in three steps.
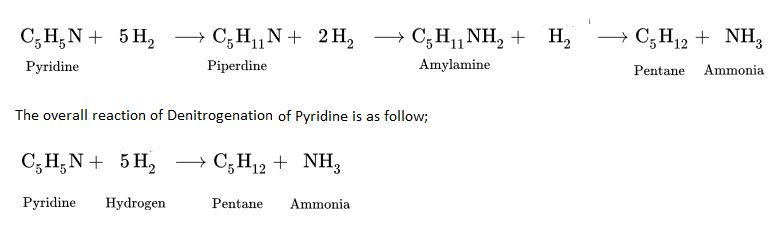
3. Oxygen Removal or Hydrodeoxygenation (HDO)
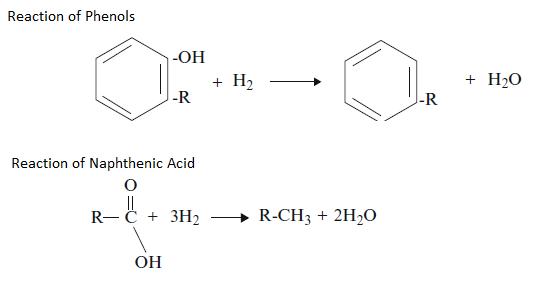
Oxygen removal, also referred to as hydrodeoxygenation, in which the organic oxygen compounds are converted to water (H2O)and corresponding Hydrocarbon by the reaction of Hydroxyl (-OH) group with the Hydrogen. Most petroleum crudes contain low levels of oxygen. The lower-molecular weight oxygen compounds can easily be removed while the higher-molecular-weight compounds – e.g., furans – are more difficult to convert. Some examples of oxygen removal are as follows;
4. Saturation of Olefins
In Olefin saturation, organic compounds containing double bonds are converted to their saturated homologues. Olefin saturation reactions are very rapid and highly exothermic. High concentrations of Olefins can cause excessive coking which can lead to pressure high drop and poor liquid flow distribution over the catalyst bed.
Diolefifins are readily hydrogenated to olefins at low temperatures. Olefins saturation reactions can be controlled by moderately reactive grading catalysts and save the bulk catalyst.
Olefins are not found in crude oils but are formed in thermal or catalytic units. Fractions containing olefins are unstable and must be protected from contact with oxygen to prevent polymer gums formation.
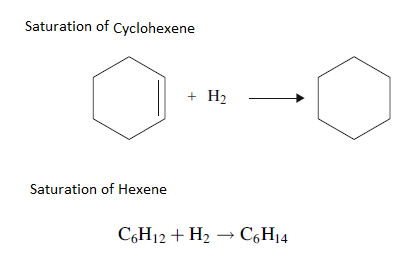
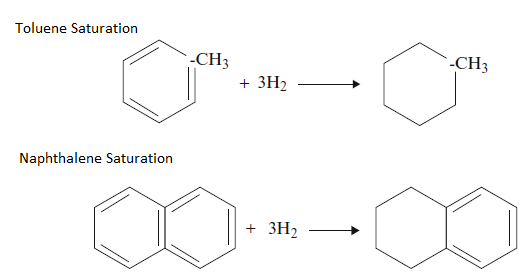
5. Saturation of aromatics or Hydrodearomatization
Aromatic saturation, also referred to as hydrodearomatization, in which some of the aromatic compounds are converted to naphthenes. Aromatics saturation is required for improvement of the quality of petroleum products, e.g., smoke point, diesel index, etc. Aromatics saturation is the most difficult reaction.
The aromatics are present as one, two, and three-ring aromatics called mono, di, and tri aromatics. The end products of aromatics saturation are naphthenic because ring opening does not occur in hydrotreating. The aromatic saturation reaction requires high hydrogen partial pressure.
6. Metals Removal or Hydrodemetallization (HDM)
Metal (organometallics) removal, also referred to as demetallation or hydrodemetallation (HDM), in which the organometallics are converted to the respective metal sulfides. Most of the metallic impurities are present in the distillates as organo-metallic compounds and occur at ppm or ppb level but the effect is significant. These metals contribute to catalyst deactivation once deposited on the catalyst pores cannot be removed by regeneration.
Most metals are arsenic, mercury, nickel, vanadium, silicon, Iron, Ca, Mg, Na, Pb, Phosphorous, etc. Na, Ca and Mg are present in the form of Inorganic compounds. Further, metals removal in hydrotreating units can be viewed in my previous blog ” Metals removal from the feed of hydrotreating units”
7. Halides Removal or Hydrodehalogenation
Halides removal, also referred to as hydrodehalogenation, in which the organic halides are converted to hydrogen halides. Organic halides, such as chlorides or bromides are present in petroleum fractions in traces. In the Hydrotreating environment, organic halides are removed by reacting with hydrogen and are converted to hydrogen halides. These then react with Ammonia and produce Ammonium chloride salts. Which are then removed with wash water addition in the downstream sections.

8. Hydrocracking Reactions (HYC)
During hydrotreatment of light and middle distillates, some hydrocracking reactions also take place. The extent of Hydrocracking increases at the end of run conditions because of high temperature. In Hydrocracking, carbon-carbon bonds are broken.
For further information, discussion and queries please comment in the box below or contact at admin@
Certified Functional Safety Professional (FSP, TÜV SÜD), Certified HAZOP & PHA Leader, LOPA Practitioner, and Specialist in SIL Verification & Functional Safety Lifecycle, with 18 years of professional experience in Plant Operations and Process Safety across Petroleum Refining and Fertilizer Complexes.
- Nasir Hussainhttps://thepetrosolutions.com/author/admin/
- Nasir Hussainhttps://thepetrosolutions.com/author/admin/
- Nasir Hussainhttps://thepetrosolutions.com/author/admin/
- Nasir Hussainhttps://thepetrosolutions.com/author/admin/







8 thoughts on “Hydrotreating Chemical Reactions”
Details about reactions is good but also show the reactions.