Chemical reactor is the heart of the processes in industries like oil refining, pharmaceuticals, fertilizers and many more. They’re the specialized vessels where raw materials transform into valuable products through controlled chemical reactions. From batch reactors for small-scale precision to continuous PFRs and CSTRs for high-volume production, each type offers unique advantages and challenges. By mastering key concepts like kinetics, thermodynamics, and mass transfer, engineers can design reactors that are safe, efficient, and innovative. Understanding how reactors work empowers you to tackle real-world challenges and drive progress in chemical engineering.
What Is a Chemical Reactor?
Chemical reactor is an equipment in which reactants are carefully mixed, heated, or cooled to create a new product. In technical terms, a chemical reactor is a vessel designed to facilitate chemical reactions under controlled conditions, such as specific temperatures, pressures, and concentrations. These conditions optimize reaction rates, maximize product yields, and ensure safety. From producing gasoline to synthesizing life-saving drugs, reactors are the backbone of countless industrial processes.
Reactors come in various shapes, sizes, and configurations, each tailored to specific reactions and industries. Their design balances chemical kinetics (how fast reactions occur) with physical processes like mixing, heat transfer, and mass transfer to achieve the desired outcome efficiently.
Why Are Chemical Reactors Important?
Reactors are critical because they determine the success of a chemical process. A well-designed reactor can:
- Boost efficiency by maximizing product yield.
- Reduce waste and energy consumption.
- Enhance safety by controlling reaction conditions to prevent hazards like runaway reactions.
- Enable innovation by supporting complex reactions for new products.
Poor reactor design can lead to inefficiencies, environmental issues, or even catastrophic incidents, making reactor knowledge vital for industry professionals and students alike.
The Basics of How Chemical Reactors Work
At their core, chemical reactors facilitate reactions by providing an environment where reactants can interact effectively. The performance of a reactor depends on several key factors:
- Reaction Kinetics: This describes how fast a reaction occurs, influenced by factors like temperature, pressure, and reactant concentration. For example, fast reactions like combustion need smaller reactors, while slow ones like wastewater treatment require larger setups.
- Mixing and Contacting: The way reactants mix affects reaction efficiency. Uniform mixing ensures consistent conditions, while controlled flow patterns (like in tubular reactors) maximize conversion.
- Mass and Heat Transfer: In reactions involving multiple phases (e.g., gas-solid), reactants must move to the reaction site, and heat must be managed to prevent temperature spikes.
- Thermodynamics: This sets the limits of a reaction’s potential. For instance, equilibrium conditions determine the maximum conversion possible, as seen in reactions like sulfur dioxide oxidation.
- Catalysts: These substances speed up reactions without being consumed, often requiring specialized reactor designs like packed beds to maximize their effect.
The relationship Output = f[input, kinetics, contacting] summarizes how a reactor’s performance depends on its inputs, reaction speed, and mixing patterns. This is called the Performance Equation of a reactor.
Types of Chemical Reactors
Chemical reactors are classified based on their operation mode, shape, and the phases involved. Below, we explore the main types and their applications, drawing from chemical engineering principles.
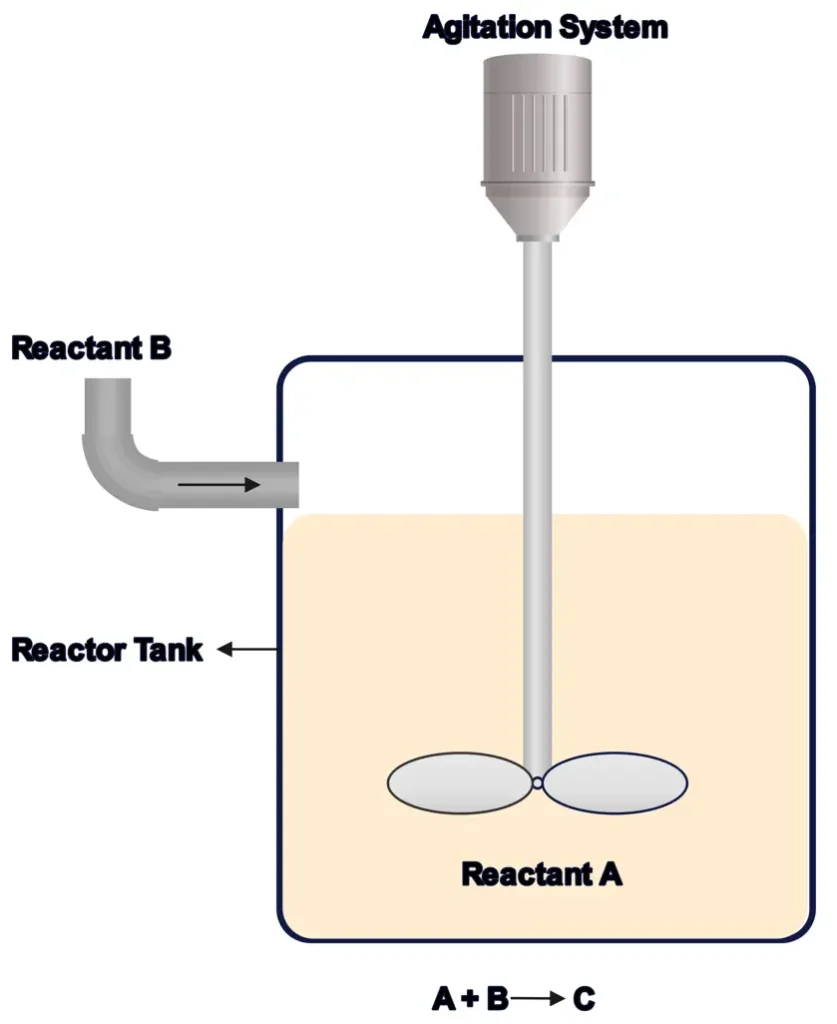
1. Batch Reactors
Batch reactors are like cooking a single batch of soup. You load all reactants at once, let the reaction run for a set time, and then remove the products. They’re ideal for small-scale or specialty processes requiring precise control.
- How They Work: Reactants are added to the reactor, mixed, and allowed to react. Once complete, the reactor is emptied and cleaned for the next batch.
- Applications: Pharmaceuticals, specialty chemicals, and fermentation (e.g., producing high-value drugs).
- Pros: Flexible, easy to clean, great for slow reactions or small-scale production.
- Cons: Labor-intensive due to loading/unloading, challenging to scale up for large production.
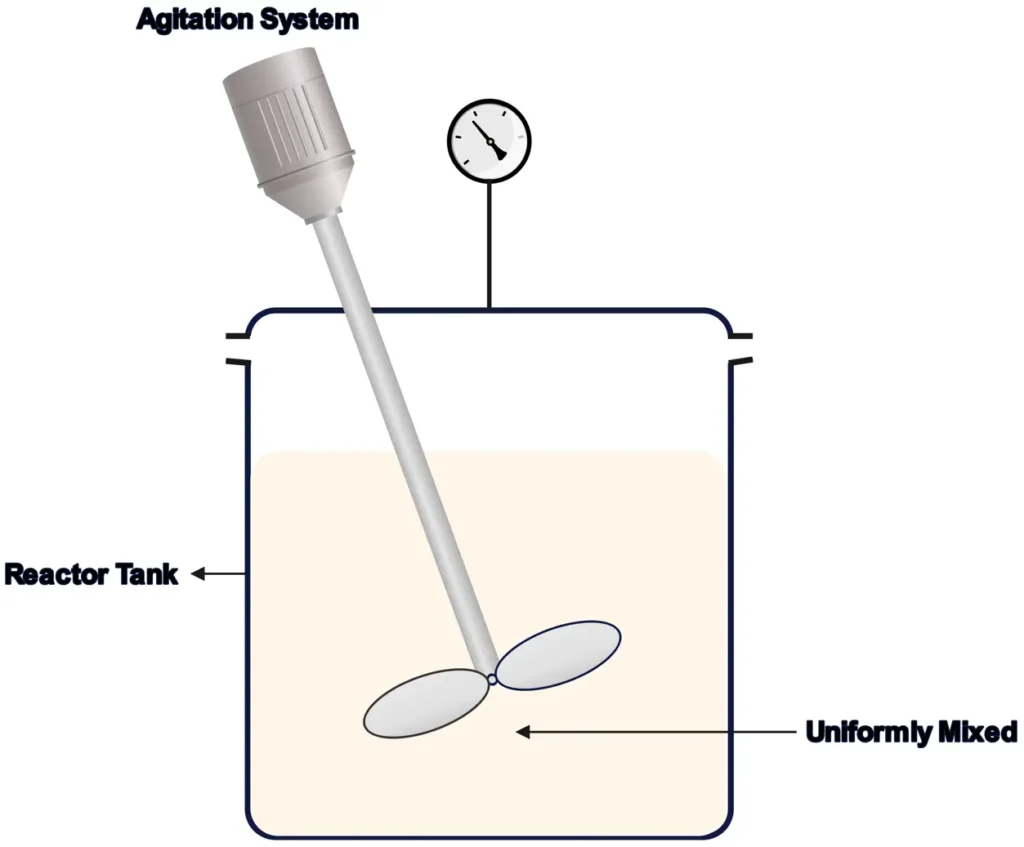
2. Continuous Stirred-Tank Reactors (CSTRs)
CSTRs, or backmix reactors, are like a blender that keeps mixing while ingredients flow in and products flow out. They maintain uniform conditions through constant stirring, making them perfect for steady-state processes.
- How They Work: Reactants enter continuously, mix thoroughly, and products exit at the same rate, ensuring consistent composition throughout.
- Applications: Large-scale chemical production, wastewater treatment, and bioprocessing (e.g., ammonia production).
- Pros: Scalable, steady output, easy to control.
- Cons: Lower conversion per volume compared to other reactors.
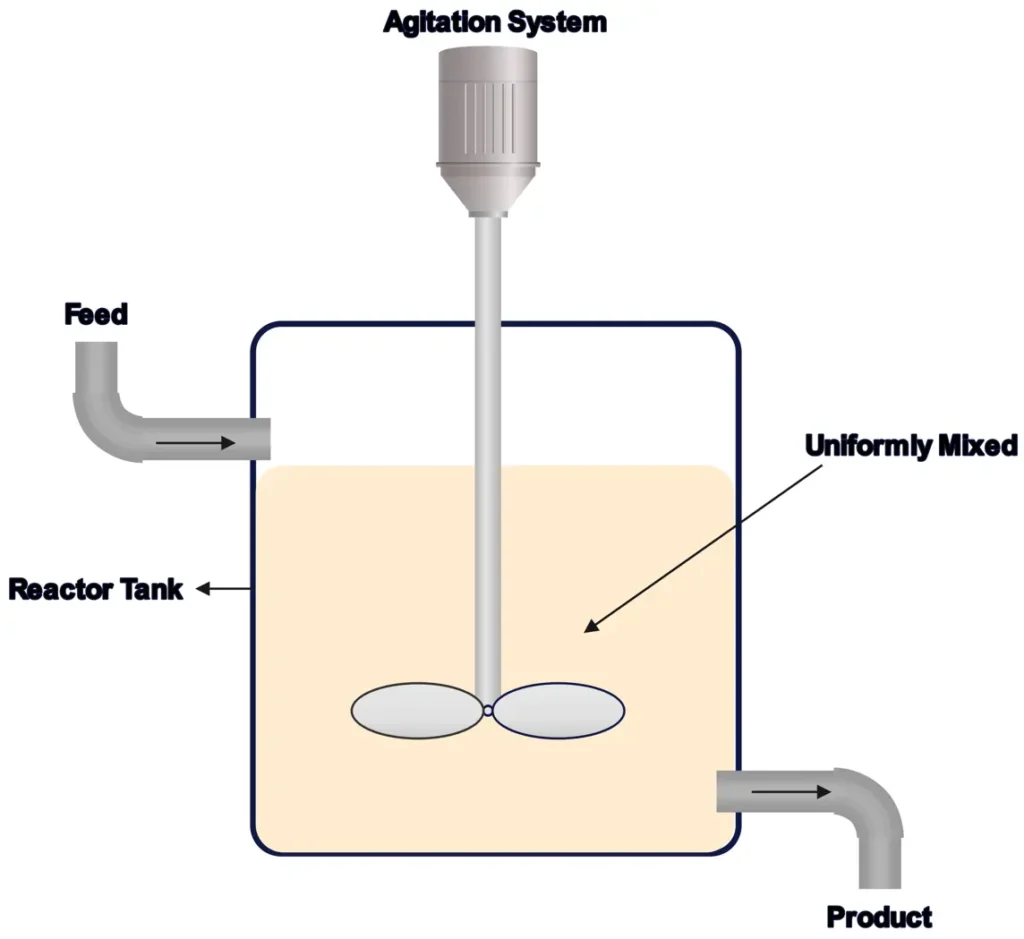
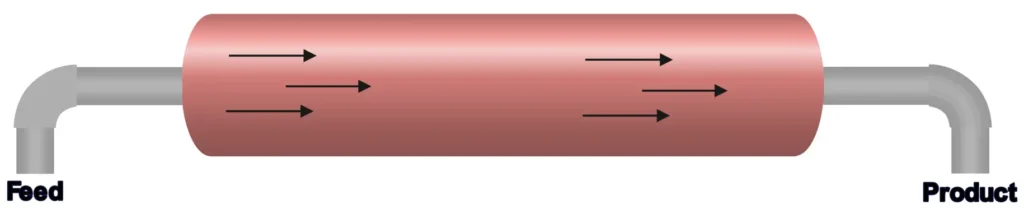
3. Plug Flow Reactors (PFRs) or Tubular Reactors
PFRs are like long pipes where reactants flow in one end, react as they move, and exit as products at the other end. They’re designed for minimal mixing along the flow path, allowing reactions to progress efficiently.
- How They Work: Reactants flow through a tube, with concentration and temperature changing along the length as the reaction occurs.
- Applications: Oil refining, gas-phase reactions, and petrochemical processes (e.g., ethylene production).
- Pros: High conversion rates, efficient for continuous processes.
- Cons: Sensitive to pressure drops, challenging to manage heat transfer.
4. Packed Bed Reactors (PBRs)
PBRs are tubular reactors filled with solid catalyst particles, ideal for reactions that rely on catalysts to speed up the process.
- How They Work: Reactants flow through a bed of catalyst, reacting as they pass, with the catalyst enhancing reaction rates.
- Applications: Catalytic cracking, ammonia synthesis, and SO₂ oxidation.
- Pros: High efficiency for catalytic reactions, scalable for industrial use.
- Cons: Catalyst deactivation (e.g., due to poisoning or coking) and pressure drop issues.

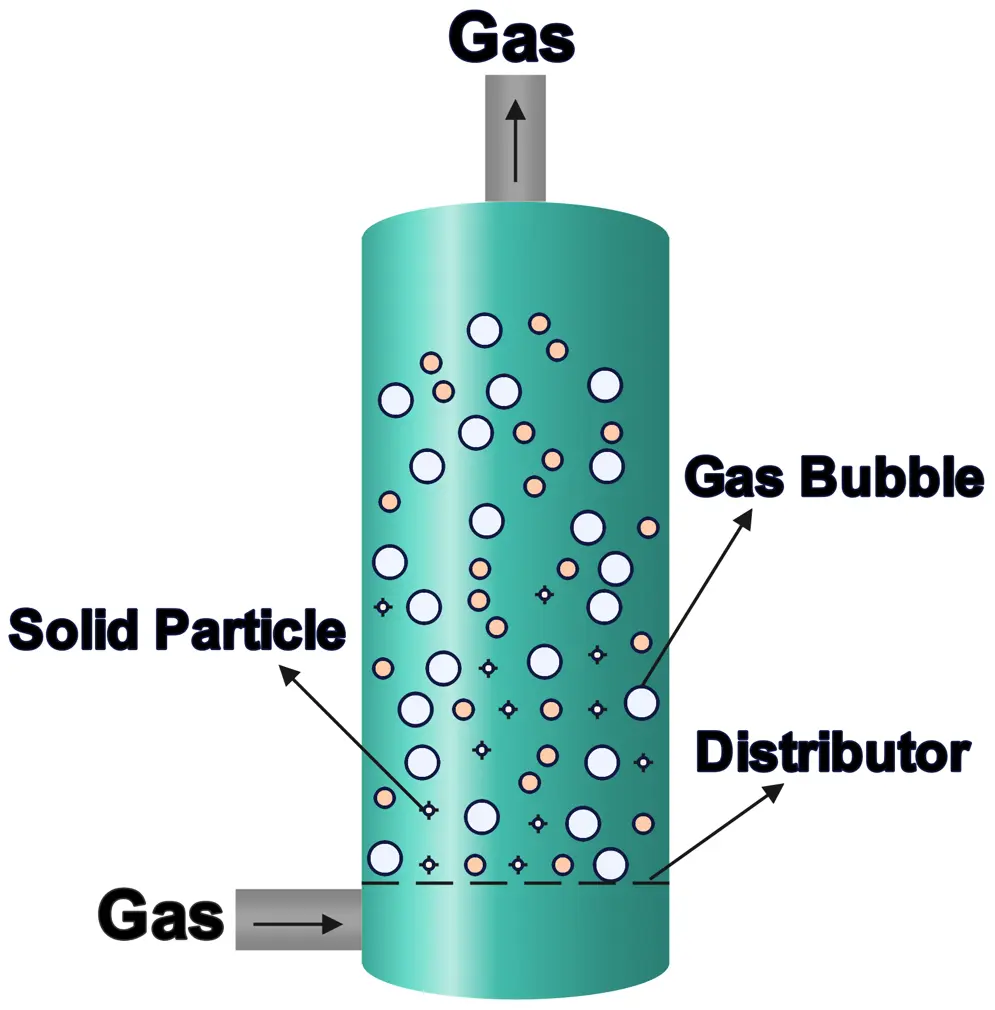
5. Fluidized Bed Reactors
Fluidized bed reactors suspend solid catalyst particles in a gas or liquid, creating a fluid-like behavior that improves mixing and heat transfer.
- How They Work: Gas or liquid flows through a bed of particles, keeping them in motion, which enhances reaction efficiency.
- Applications: Fluid catalytic cracking (FCC) in refineries, acrylonitrile production.
- Pros: Excellent heat and mass transfer, suitable for large-scale processes.
- Cons: Complex design, potential for catalyst attrition.
6. Semibatch Reactors
Semibatch reactors blend batch and continuous features, where some reactants are loaded initially, and others are added gradually during the reaction.
- How They Work: One reactant is charged at the start, while others are fed continuously to control reaction rates.
- Applications: Polymerization, controlled exothermic reactions.
- Pros: Flexible, allows better control over reaction conditions.
- Cons: Complex operation, higher operational costs.
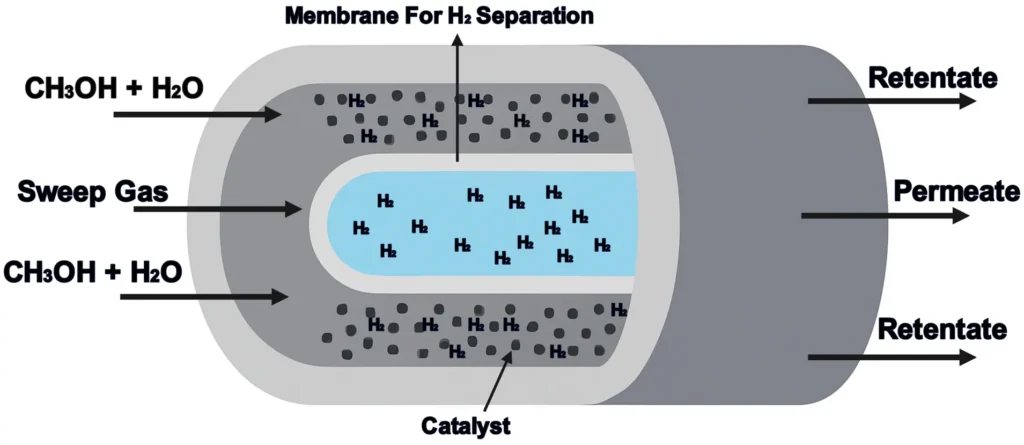
7. Membrane Reactors
Membrane reactors use selective membranes to separate products or feed reactants, improving reaction selectivity and yield.
- How They Work: A membrane allows specific molecules to pass, enhancing the efficiency of the reaction.
- Applications: Hydrogen production, selective oxidation.
- Pros: Improved yield and selectivity.
- Cons: Expensive, risk of membrane fouling.
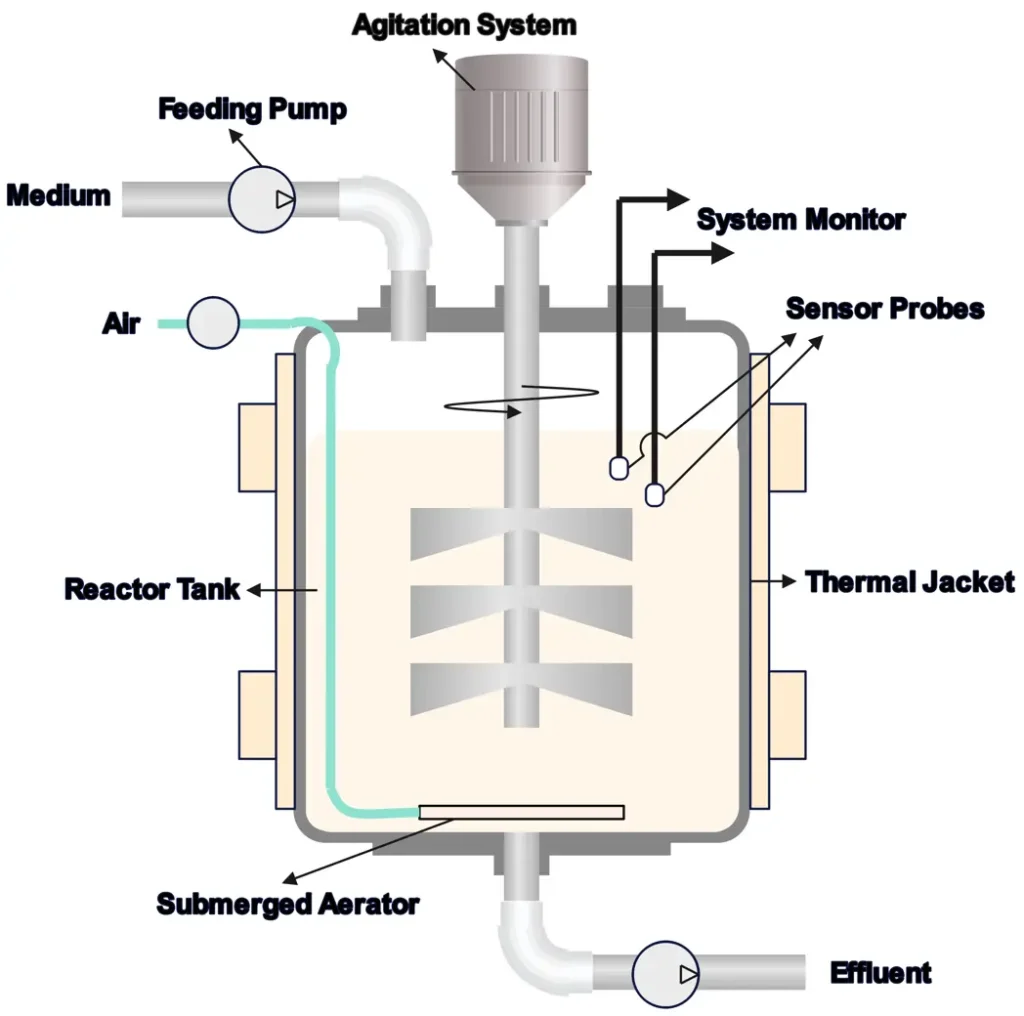
8. Bioreactors
Bioreactors are tailored for biological reactions, often involving microorganisms or enzymes, and require precise control of conditions like pH and oxygen levels.
- How They Work: Microbes or enzymes catalyze reactions in a controlled environment, often in batch or continuous setups.
- Applications: Fermentation for biofuels, antibiotics, or insulin production.
- Pros: Supports complex biological processes.
- Cons: Sensitive to contamination, challenging to scale.
People Also Asked Questions with Answers
What is the difference between batch and continuous reactors?
Batch reactors process a fixed amount of reactants at once, with no flow in or out during the reaction, making them ideal for small-scale or flexible production like pharmaceuticals. Continuous reactors, like CSTRs or PFRs, have a steady flow of reactants and products, suited for large-scale, consistent production, such as in oil refining.
Why is mixing important in reactors?
Mixing ensures uniform contact between reactants, improving reaction efficiency and preventing issues like hot spots or uneven reactions. In CSTRs, thorough mixing maintains consistent conditions, while in PFRs, controlled flow patterns optimize conversion.
How do catalysts affect reactor design?
Catalysts speed up reactions without being consumed, allowing smaller reactors or lower operating temperatures. However, they can deactivate due to poisoning or fouling, requiring specialized designs like packed or fluidized beds to maximize efficiency and manage catalyst life.
What is residence time distribution (RTD)?
RTD measures how long fluid elements stay in a reactor, helping engineers understand mixing and flow patterns. It’s crucial for analyzing non-ideal flow and optimizing reactor performance, especially in continuous systems.
References
- Nauman, E. B. (2002). Chemical Reactor Design, Optimization, and Scaleup. McGraw-Hill.
- Hill, C. G., & Root, T. W. (2014). Introduction to Chemical Engineering Kinetics and Reactor Design. Wiley.
- Froment, G. F., & Bischoff, K. B. (1979). Chemical Reactor Analysis and Design. John Wiley & Sons Inc.
- Levenspiel, O. (1999). Chemical Reaction Engineering. Wiley.
- Fogler, H. S. (n.d.). Elements of Chemical Reaction Engineering.
- https://www.sciencedirect.com
- https://microbenotes.com





