Unit operations and unit processes are the building blocks of turning raw materials into finished products, like transforming crude oil into gasoline or sugar cane into refined sugar. Unit operations handle the physical tasks (moving, heating, or separating materials) while unit processes dive into the chemistry, creating new substances through reactions. Together, they’re the heart of chemical engineering, making production efficient, safe, and scalable. From refining crude oil to life-saving pharmaceuticals, these steps solve challenges with precision. This blog breaks down how they work, with clear examples from industries.
What Are Unit Operations and Unit Processes?
Unit operations and unit processes are the steps that transform raw material into a finished product.
- Unit Operations: These are the physical steps that move, shape, or separate materials without changing their chemical makeup. Pumping liquids through pipes, heating a mixture, or filtering out solids are all unit operations. Examples include distillation, filtration, drying, and evaporation. These are the mechanical moves that set the stage for chemical reactions or polish the final product.
- Unit Processes: These involve chemical reactions that transform the molecular structure of materials, creating entirely new substances. Think of alkylation turning raw hydrocarbons into high-octane gasoline or hydrogenation saturating oils to make margarine. These are the chemistry-driven steps that give materials their new identity.
Together, unit operations and unit processes form the backbone of chemical engineering, allowing engineers to design systems that are efficient, safe, and scalable.
Why Do They Matter?
Unit operations and unit processes are like the gears of a well-oiled machine. They allow engineers to:
- Streamline Production: Break down complex processes into manageable steps, making it easier to design and optimize systems.
- Ensure Safety: Identify potential hazards and implement safety measures, like emergency shutdowns, to comply with regulations.
- Boost Efficiency: Optimize material and energy use to cut costs and reduce environmental impact.
- Solve Problems: From neutralizing hazardous waste to producing high-quality pharmaceuticals, these concepts tackle real-world challenges.
Unit Operations: The Physical Side
Unit operations are all about physical transformations such as moving, heating, or separating materials to prepare them for reactions or refine them into products. Let’s explore a few key ones with practical examples.
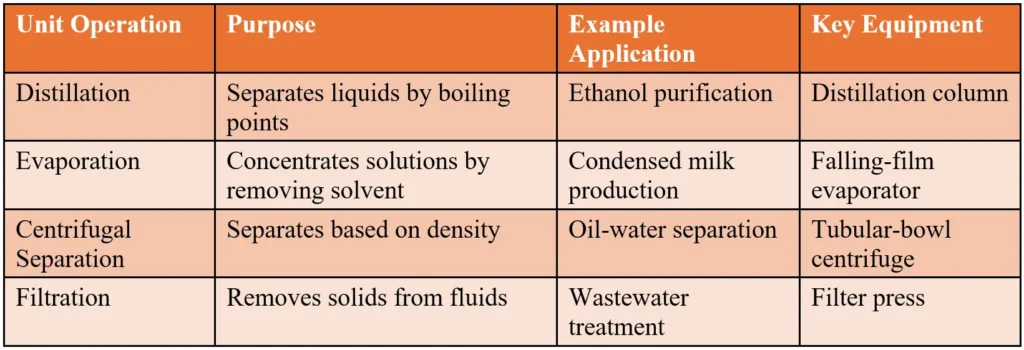
1. Distillation
Distillation separates liquids based on their boiling points. In a petroleum refinery, crude oil is heated in a distillation column, separating it into fractions like gasoline, kerosene, and diesel.
2. Evaporation
Evaporation concentrates solutions by removing solvents (usually water) as vapor. It’s like boiling down soup to make it thicker. In the food industry, it turns milk into condensed milk, while in chemical plants, it concentrates sodium hydroxide.
3. Centrifugal Separation
Centrifugal separation uses high-speed rotation to separate materials based on density. It’s used to clarify liquids (e.g., removing solids from vegetable oil) or separate liquids (e.g., oil from water in refining).
4. Filtration
Filtration removes solids from liquids or gases using media like fabric or membranes. It’s crucial for purifying wastewater or air.
Unit Processes: The Chemical Side
Unit processes involve chemical reactions that transform materials into new substances. Here are some key examples.
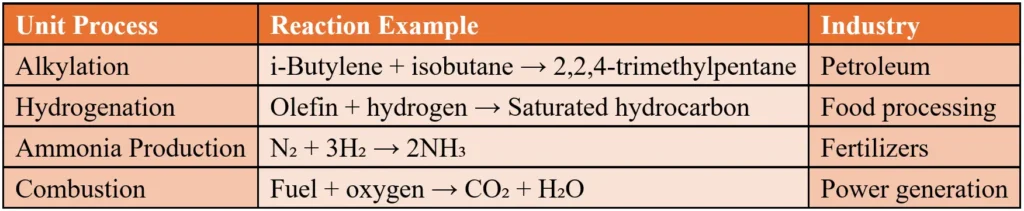
1. Alkylation
Alkylation (an alkyl group is added to a molecule) combines molecules to create larger ones, like producing high-octane gasoline in petroleum refining.
2. Hydrogenation
Hydrogenation adds hydrogen to molecules, often to saturate compounds. It’s used in food processing (e.g., turning oils into margarine) and oil refining.
3. Ammonia Production
Ammonia is produced via the reaction: N₂ + 3H₂ → 2NH₃. Recycling unreacted gases improves efficiency.
4. Combustion
Combustion is an exothermic reaction where fuel reacts with oxygen, producing heat and gases like CO₂ and H₂O.
How Both Work Together
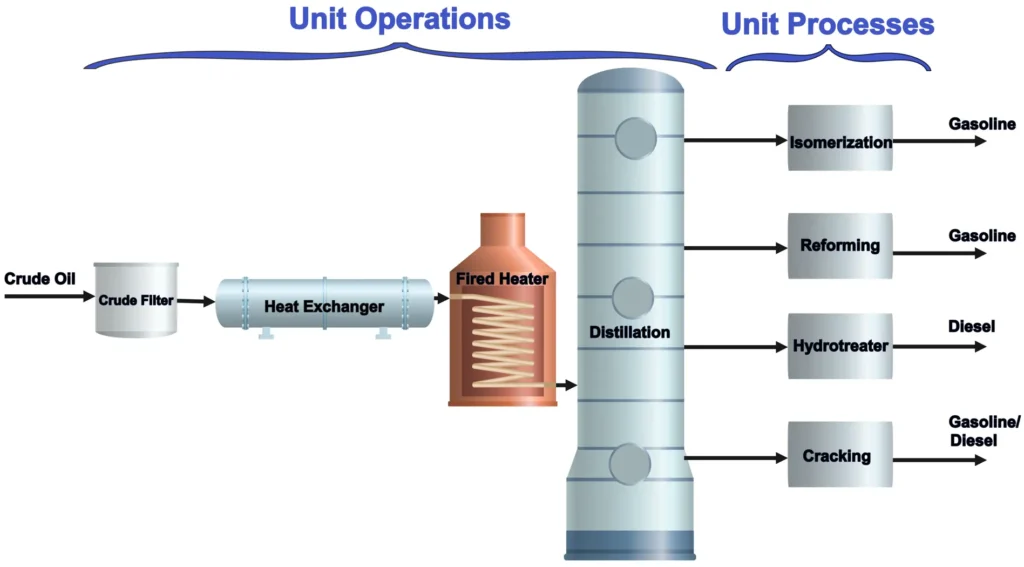
They work together in industrial systems to convert raw materials into desired products efficiently. Below are examples illustrating how they integrate and their roles:
Petroleum Refining
- Industry: Oil and Gas
- Process Overview: Crude oil is refined into usable products like gasoline, diesel, and kerosene through a combination of physical separations and chemical transformations.
- Unit Operations:
- Distillation: Crude oil is heated in a distillation column, separating it into fractions based on boiling points. The crude oil is heated to vaporize lighter components, which rise and condense at different heights in the column.
- Filtration: Removes solid impurities or particulates from crude oil before processing.
- Heat Exchange: Heats crude oil before distillation and cools fractions after separation to optimize energy use.
- Unit Processes:
- Catalytic Cracking: Heavy fractions (e.g., gas oil) are broken into lighter molecules (e.g., gasoline) using a catalyst at high temperatures.
- Hydrotreating: Removes sulfur impurities by reacting the oil with hydrogen over a catalyst, producing hydrogen sulfide and cleaner fuel.
Crude oil is first preheated (unit operation: heat exchanger) and separated into fractions (unit operation: distillation). Heavy fractions undergo catalytic cracking (unit process) to produce gasoline. The products are then purified through additional distillation or filtration (unit operations) to meet quality standards. Hydrotreating (unit process) ensures low sulfur content, followed by cooling (unit operation) for storage.
Pharmaceutical Manufacturing
- Industry: Pharmaceutical (Aspirin Production)
- Process Overview: Aspirin (acetylsalicylic acid) is synthesized from salicylic acid and purified for commercial use.
- Unit Operations:
- Mixing: Salicylic acid is mixed with acetic anhydride in a reactor to ensure uniform reaction conditions.
- Crystallization: After synthesis, aspirin is crystallized from the reaction mixture by cooling or adding a solvent to form pure crystals.
- Filtration and Drying: Crystals are filtered from the liquid and dried to remove residual solvents.
- Unit Processes:
- Acetylation: Salicylic acid reacts with acetic anhydride in the presence of a catalyst (e.g., sulfuric acid) to form acetylsalicylic acid (aspirin) at around.
Salicylic acid and acetic anhydride are mixed (unit operation) to prepare for the acetylation reaction (unit process), which forms aspirin. The resulting mixture is cooled to induce crystallization (unit operation), and the aspirin crystals are filtered and dried (unit operations) for packaging.
Food Processing
- Industry: Sugar Production
- Process Overview: Raw sugar cane juice is processed into refined white sugar through physical and chemical steps.
- Unit Operations:
- Extraction: Sugar cane is crushed, and juice is extracted using rollers or presses.
- Filtration: Juice is filtered to remove plant debris and impurities.
- Evaporation: The juice is heated to evaporate water, concentrating the sugar solution.
- Crystallization: The concentrated solution is cooled to form sugar crystals.
- Unit Processes:
- Liming: Calcium hydroxide is added to the juice to neutralize acids and precipitate impurities, forming insoluble compounds.
- Carbonation: Carbon dioxide is bubbled through the juice to remove excess lime as calcium carbonate, which is filtered out.
Sugar cane juice is extracted (unit operation) and treated with lime (unit process) to remove impurities. The juice is filtered (unit operation) and concentrated through evaporation (unit operation). Carbonation (unit process) further purifies the solution, followed by crystallization and filtration (unit operations) to produce refined sugar.
People Also Asked Questions with Answers
What is the difference between a unit operation and a unit process?
A unit operation involves physical changes (e.g., distillation, filtration) without altering the chemical composition of materials. A unit process involves chemical reactions (e.g., alkylation, hydrogenation) that change the molecular structure.
Why are unit operations important?
Unit operations are critical because they provide standardized methods to handle materials physically, ensuring efficient, safe, and scalable production processes.
What are some examples of unit processes?
Alkylation (gasoline production), hydrogenation (food processing), and ammonia synthesis (fertilizers) are common examples.
How do unit operations and unit processes improve process safety?
By breaking processes into manageable steps, engineers can identify hazards, optimize conditions, and implement safety protocols like emergency shutdowns.
References:
- Dryden, C. E. (n.d.). Outlines of Chemical Technology.
- Solen, K. A., & Harb, J. N. (2011). Introduction to Chemical Engineering: Tools for Today and Tomorrow. Wiley.
- Ghasem, N., & Henda, R. (2025). Principles of Chemical Engineering Processes: Material and Energy Balances. CRC Press.
- Geankoplis, C. J. (1993). Transport Processes and Unit Operations. Prentice Hall.
- https://www.haberwater.com
- https://www3.epa.gov