Kerogen in petroleum is a complex fossilized organic material, found in the oil shale and other sedimentary rock, which is insoluble in common organic solvents and yields petroleum products on heating. Kerogen forms the precursor to hydrocarbons in sedimentary rocks, including oil and natural gas. It is derived from the remains of ancient organic matter, such as algae, woody plant material, plankton, and other microorganisms that have been deposited in sedimentary basins over millions of years.
Kerogen has a high molecular weight relative to tar sand and is used to produce oil and gas. Kerogen is essentially a mixture of complex organic compounds that are not yet fully matured into crude oil or natural gas but can be converted into these resources through thermal and chemical processes to produce shale oil and gas.
How Kerogen is Formed?
Kerogen is formed through a process called diagenesis, which involves the transformation of organic matter in sedimentary rocks due to pressure and temperature changes over millions of years. The process of kerogen formation begins with the deposition of organic matter, such as dead plants and animals, in sedimentary rocks like shale or mudstone. As sediment accumulates on top of this organic material, it becomes buried deeper and deeper beneath the Earth’s surface.
Over time, the temperature and pressure in the surrounding sediment increase due to the heat generated by the Earth’s core and the weight of the overlying rock. As a result, the organic matter undergoes a series of chemical changes, including dehydration, decarboxylation, and polymerization. These chemical changes lead to the formation of kerogen.
Ultimately, if the temperature and pressure conditions are sufficient, the kerogen may undergo further thermal maturation and transformation into hydrocarbons like oil and gas. However, not all kerogen will transform into hydrocarbons, and the formation of oil and gas deposits requires a complex interplay of geological and chemical processes over millions of years.
Types and Characteristics of Kerogen
Kerogen has a high molecular weight relative to tar sand bitumen and is generally insoluble in typical organic solvents. There are four main types of kerogen, known as Types I, II, III, and IV.
Type I Kerogen (Lacustrine Algal)
Type I Kerogen consists of marine algae and amorphous constituents which are typically found in sedimentary rocks that were deposited in anoxic environments, such as deep marine basins. It is rich in hydrogen and produces the largest quantity of oil as compared to other types when heated. The general properties of Type-I Kerogen are as follows;
- Hydrogen/carbon atomic ratio >1.25.
- Oxygen/carbon atomic ratio <0.15.
- Tendency to rapidly produce liquid hydrocarbons.
- Are derived principally from lacustrine algae.
- Has few cyclic or aromatic structures.
- Formed mainly from proteins and lipids.
- Rich in lipid-derived aliphatic chains and has a relatively low content of polynuclear aromatic systems and heteroatomic systems.
- Organic sources for the type I kerogen include the lipid-rich products of algal blooms and the finely divided
and extensively reworked lipid-rich biomass deposited in stable stratified lakes.
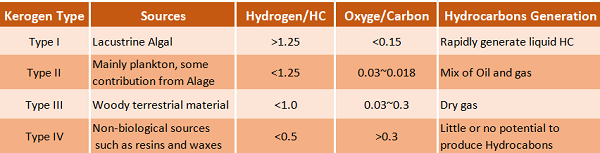
Type II Kerogen (Planktonic)
Type II is derived from mixed terrestrial, plankton and other marine organisms, and is found in rocks that were deposited in marine or lacustrine environments. The organic matter in this type of kerogen is usually derived from a mixture of zooplankton, phytoplankton, and bacterial remains that were deposited in a reducing environment is also rich in hydrogen but produces a mix of oil and gas when heated. The general properties of Type II Kerogen are as follows;
- Hydrogen/carbon atomic ratio <1.25.
- Oxygen/carbon atomic ratio 0.03 to 0.18.
- Tends to produce a mix of gas and oil.
- Several types: exinite, cutinite, resinite, and liptinite.
- Exinite is formed from pollen and spores.
- Cutinite is formed from terrestrial plant cuticle.
- Resinite is terrestrial plant resins, animal decomposition resins.
- Liptinite is formed from terrestrial plant lipids and marine algae.
Type III Kerogen is characteristic of coals and coaly shales. Easily identified fossilized plants and plant fragments are common, indicating that this type of kerogen is derived from woody terrestrial material. It is rich in carbon and produces primarily gas when heated.
- Hydrogen/carbon atomic ratio <1.0.
- Oxygen/carbon atomic ratio 0.03 to 0.3.
- The material is thick, resembling wood or coal.
- Tends to produce coal and gas.
- Has very low hydrogen content because of the extensive ring and aromatic systems.
- Formed from terrestrial plant matter that is lacking in lipids or waxy matter; forms from cellulose, the carbohydrate polymer that forms the rigid structure of terrestrial plants, lignin, another carbohydrate polymer (polysaccharide) that binds the strings of cellulose together, and terpenes and phenolic compounds in the plant.
Type IV Kerogen
Type IV is derived from non-biological sources, such as resins and waxes, and is found in rocks that were deposited in arid environments. Type IV Kerogen consists mostly of decomposed organic matter in the form of polycyclic aromatic hydrocarbons and has the following properties;
- Low Hydrogen/carbon atomic ratio <0.5.
- Contains mostly decomposed organic matter in the form of polycyclic aromatic hydrocarbons.
- Little or no potential to produce hydrocarbons
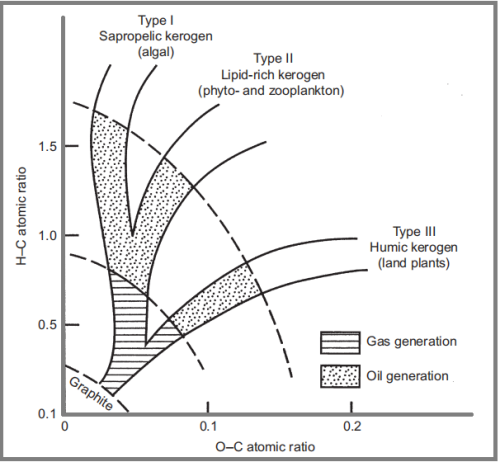
The Van Krevelen diagram as shown below (a plot of the atomic hydrogen-carbon ratio versus the atomic oxygen-carbon ratio), derived from the elemental analysis of kerogen and coal is a practical means of studying kerogen composition and properties.
Kerogen plays a critical role in the formation of oil and natural gas. As sedimentary rocks are buried and heated over time, the kerogen undergoes a series of thermal and chemical reactions, leading to the formation of hydrocarbons. The exact process depends on the type and quality of the kerogen, as well as the temperature and pressure conditions. In general, the kerogen breaks down into smaller hydrocarbon molecules, which are then expelled from the rock and migrate into reservoirs where they can be extracted.
Understanding the properties and distribution of kerogen is essential for the exploration and production of oil and natural gas. Geologists and petroleum engineers use a variety of methods to characterize kerogen in rocks, including geochemical analysis, petrographic microscopy, and thermal maturation studies. This information can be used to predict the quality and quantity of hydrocarbons that may be present in a given rock formation.
Top References
- Applied source rock geochemistry by Peters, K. E., & Cassa, M. R. (1994).AAPG Bulletin, 78(1), 38-81.
- Petroleum formation and occurrence (Vol. 2) by Tissot, B. P., & Welte, D. H.
- Shale Oil Production Processes by James G. Speight
For further information, discussion and queries please comment in the box below or contact us at admin@ or follow us on Facebook & LinkedIn.