Hydrocracking is a catalytic hydrogenation process in which high molecular weight feedstocks are converted and hydrogenated to lower molecular weight products. Cracking will break bonds, and the resulting unsaturated products are consequently hydrogenated into stable compounds. Hydrogenation also removes impurities such as sulfur, nitrogen, and metals in the feed. The objective of the hydrocracking process is the transformation of the low-valued heavy fractions of crude oil into light high-priced petroleum products.
Two distinct types of catalytic sites are required to catalyze the cracking reaction sequence. One of the functions is called the acid function, which provides cracking and isomerization activity, and the second function is the metal function, which provides olefin formation and hydrogenation/saturation activity.
The acidic function of hydrocracking catalysts is provided by the support which is usually an amorphous oxide such as silica-alumina and zeolite. Silica-alumina support is common for producing higher yields of middle distillate while zeolites have higher activity and better selectivity to gasoline. The metal function for hydrocracking may be obtained from a combination of Group VIA (Mo, W) and Group VIIA (Co, Ni) metal sulfides.

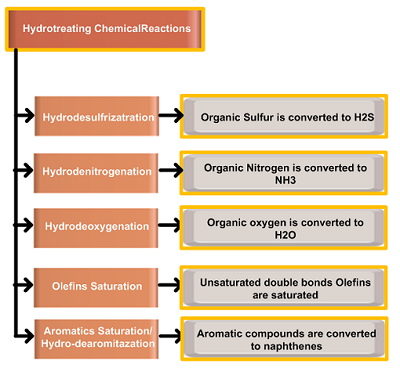
There are two types of reactions taking place in a catalytic environment, hydrotreating and hydrocracking reactions. Both hydrotreating and hydrocracking reactions can proceed in a single reactor or in a separate reactor followed by a hydrocracking reactor. The initial reactions in the hydrocracking process are therefore hydrotreating reactions and occur at a relatively high rate over a hydrotreating catalyst. Hydrocracking catalysts will also remove any residual sulfur and nitrogen remaining after hydrotreating. The details of hydrotreating reactions can be viewed in my previous blog “Hydrotreating Chemical Reactions“.
Hydrocracking reactions
In hydrocracking units, cracking reactions i.e. breaking carbon to carbon bonds can be grouped into three main categories: 1. Paraffin hydrocracking, 2. Naphthene ring-opening, 3. Dealkylation of aromatics and naphthenic rings.
Organic nitrogen compounds suppress the activity of hydrocracking catalysts by adsorbing strongly to the acid sites. Therefore, the organic nitrogen content in feed must be greatly minimized before hydrocracking in one or more beds of catalysts in the reactor. Further, most of the ammonia is removed by injecting wash water in the reactor circuit. Hydrodenitogenation converts organic nitrogen into ammonia, which also adsorbs to the acid sites, affecting the activity of the catalyst.
1. Paraffins Hydrocracking
This is the most important hydrocracking reaction in the unit, that breaks the large hydrocarbon paraffin into smaller saturated hydrocarbons. This reaction follows the following path;
A. Adsorption of a paraffin molecule to a metal site, dehydrogenation of paraffin hydrocarbon to form an olefin
B. The olefin forms a carbonium ion on an acid site.
C. Isomerization and hydrocracking of the carbonium ion produce an olefin and a smaller carbonium ion. The olefin can undergo further cracking on an acid site, or it can react with hydrogen at a metal site.
D. Hydrogen addition at the metal site to form a saturated iso-paraffin.
E. The carbonium ion in Step C is converted to paraffin via deprotonation.

The overall short reaction is shown below, where large Paraffin Hydrocarbon is divided into two smaller groups, R & R1.

2. Dealkylation of Aromatics
The Alkyl group is removed from the Aromatics and forms a stable compound. Hydrocracking of alkylbenzenes with side chains of three to five carbon atoms gives relatively simple products. The larger the alkyl side chain, the more complex the product distribution.

3. Naphthenes Ring Opening
The main reactions of naphthenes with a single five-membered or six-membered ring are skeletal isomerization and hydrocracking, similar to those observed for n-paraffins but more difficult than paraffins hydrocracking.

4. Hydrocracking of Polynuclear Aromatics Groups
The reaction mechanism of hydrocracking polycyclic aromatics is fairly complex and involves hydrogenation, isomerization, alkylation, and cracking.

5. Hydroisomerization
Isomers formation is the intermediate of the hydrocracking process, where paraffin molecule is converted into the branched-chain molecule. At low temperatures and low degrees of conversion, the hydroisomerization of n-paraffins predominates. As the temperature increases, the degree of hydroisomerization goes through a maximum and starts decreasing, whereas the degree of hydrocracking increases. The decrease in hydroisomerization at higher temperatures is due to the consumption of branched isomers by hydrocracking. Cracking and isomerization reactions take place on the acidic support.

For further information, discussion and queries please comment in the box below or contact at admin@ or follow us on Facebook & LinkedIn.
Certified Functional Safety Professional (FSP, TÜV SÜD), Certified HAZOP & PHA Leader, LOPA Practitioner, and Specialist in SIL Verification & Functional Safety Lifecycle, with 18 years of professional experience in Plant Operations and Process Safety across Petroleum Refining and Fertilizer Complexes.
- Nasir Hussain
- Nasir Hussain
- Nasir Hussain
- Nasir Hussain
- Nasir Hussain
- Nasir Hussain






