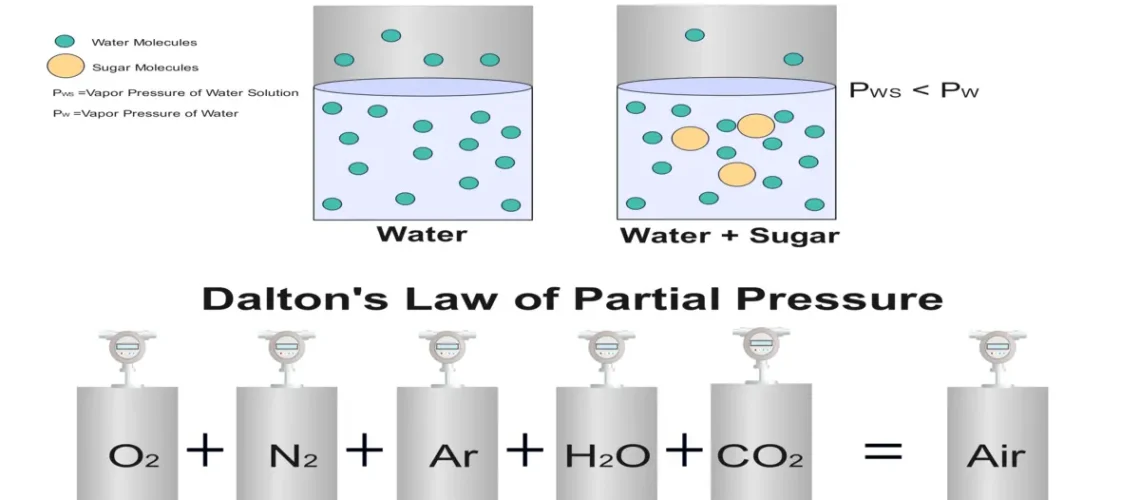
In the world of chemistry, understanding how substances behave in mixtures is interesting. Have you ever wondered what happens at the molecular level when liquids turn into vapors or how gases mix in a container? The answers lie in two fundamental principles of chemistry. Raoult’s Law and Dalton’s Law of Partial Pressures. These laws unlock the secrets of vapor pressure and gas behavior, guiding everything from industrial processes to laboratory experiments. In this blog, we’ll dive deep into both laws, exploring their definitions, formulas, applications, and the fascinating interplay between them.
What is Raoult’s Law? A Simple Definition
Raoult’s Law, named after French chemist François-Marie Raoult, describes how the vapor pressure of a solvent in a solution is influenced by the presence of a solute. In simple terms, it says that the vapor pressure of a solvent in a mixture is proportional to its mole fraction in that mixture. Imagine you’re mixing sugar into water—the sugar molecules reduce the number of water molecules escaping into the vapor phase, lowering the vapor pressure. This principle is key in understanding solutions, especially in chemical engineering and process industries.
Raoult’s Law Statement: The partial vapor pressure of a component in a solution is equal to the vapor pressure of the pure component multiplied by its mole fraction in the solution.
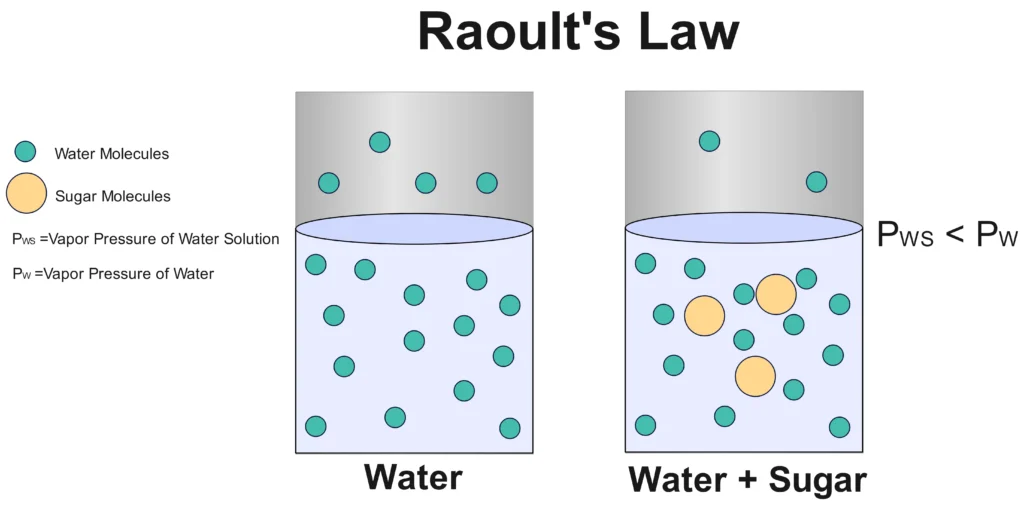
Raoult’s Law Formula
The mathematical expression for Raoult’s Law is straightforward:
![]() Where:
Where:

For a solution with multiple volatile components (say, liquids A and B), Raoult’s Law applies to each:
![]()
The total vapor pressure is the sum of these partial pressures, which brings us to a connection with Dalton’s Law.
Applications of Raoult’s Law in the Real World
Raoult’s Law is a basic principle used in chemical engineering and process industries. Here are some key applications:
- Distillation Processes: In petroleum refineries, Raoult’s Law helps predict how components in crude oil will separate based on their vapor pressures during distillation.
- Vapor Pressure Lowering: Used in designing solutions for chemical reactors, where controlling vapor pressure is critical for safety and efficiency.
- Colligative Properties: Raoult’s Law explains properties like boiling point elevation and freezing point depression, vital for formulating antifreeze or desalination processes.
- Pharmaceuticals: Helps in preparing solutions with precise vapor pressures for drug manufacturing.
For example, in a refinery, engineers use Raoult’s Law to calculate the vapor pressure of a gasoline blend, ensuring it meets safety standards for storage and transport.
What is Dalton’s Law of Partial Pressures?
Now, let’s move to Dalton’s Law of Partial Pressures, formulated by John Dalton in 1801. This law applies to mixtures of non-reacting gases, stating that the total pressure of a gas mixture is the sum of the partial pressures of each individual gas. Each gas behaves as if it were alone in the container, contributing its own pressure based on its mole fraction.
Dalton’s Law Definition: The total pressure exerted by a mixture of non-reacting gases is equal to the sum of the partial pressures of each gas in the mixture.
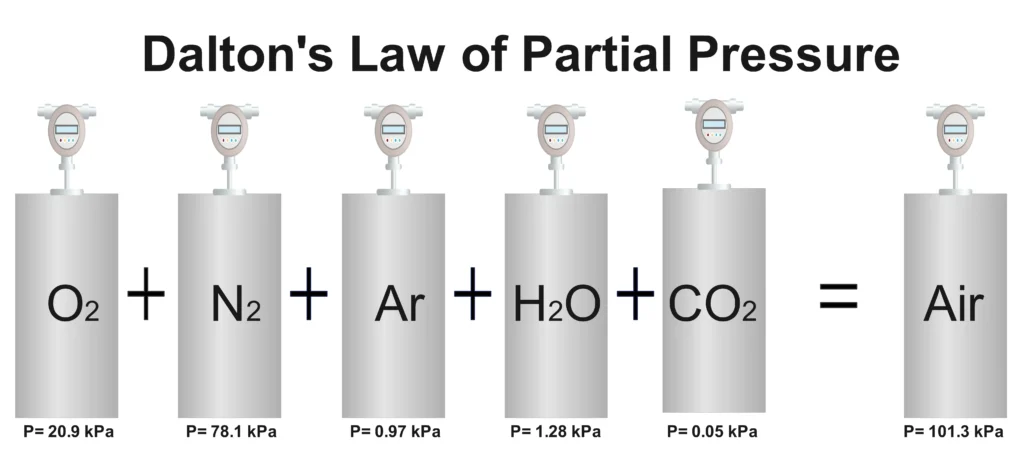
Dalton’s Law Formula
Mathematically, Dalton’s Law is expressed as:
![]()
Where:
![]()
The partial pressure of a gas is related to its mole fraction:
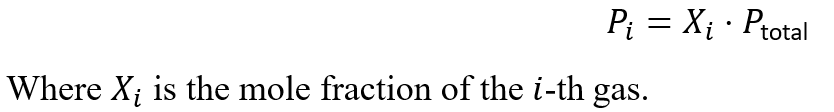
Examples of Dalton’s Law in Action
- Atmospheric Pressure: The total atmospheric pressure is the sum of partial pressures of nitrogen, oxygen, carbon dioxide, and other gases.
- Scuba Diving: The air in a scuba tank is a mixture of oxygen, nitrogen, and other gases. Dalton’s Law helps calculate the partial pressure of oxygen to ensure safe breathing at different depths.
- Industrial Gas Mixtures: In a chemical plant, a reactor might contain a mix of hydrogen, nitrogen, and argon. Dalton’s Law allows engineers to determine the contribution of each gas to the total pressure, ensuring safe operating conditions.
Applications of Dalton’s Law
Dalton’s Law is widely used in process industries and beyond:
- Gas Storage and Transport: Ensures safe handling of gas mixtures in cylinders, like those used in welding or medical applications.
- Environmental Engineering: Helps measure partial pressures of gases like CO₂ in air quality monitoring.
- Chemical Reactors: Used to predict gas behavior in reactions where multiple gases are present, ensuring process safety.
- Anesthesia: In medical settings, Dalton’s Law guides the preparation of gas mixtures for anesthesia, balancing partial pressures for patient safety.
Difference Between Raoult’s Law and Dalton’s Law
While both laws deal with pressures, they apply to different systems:
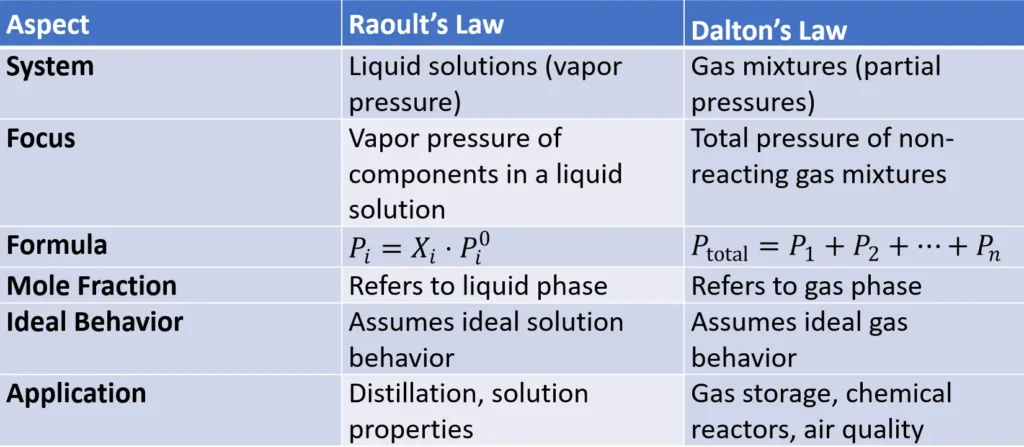
In short, Raoult’s Law governs the vapor pressure of liquids in a solution, while Dalton’s Law deals with the pressures of gases in a mixture.
Relationship Between Raoult’s Law and Dalton’s Law
Raoult’s and Dalton’s Laws are closely related, especially when analyzing the vapor phase above a liquid solution. Raoult’s Law calculates the partial vapor pressure of each component in the liquid phase, while Dalton’s Law sums these partial pressures to find the total vapor pressure above the solution. For an ideal solution with two volatile liquids A and B:
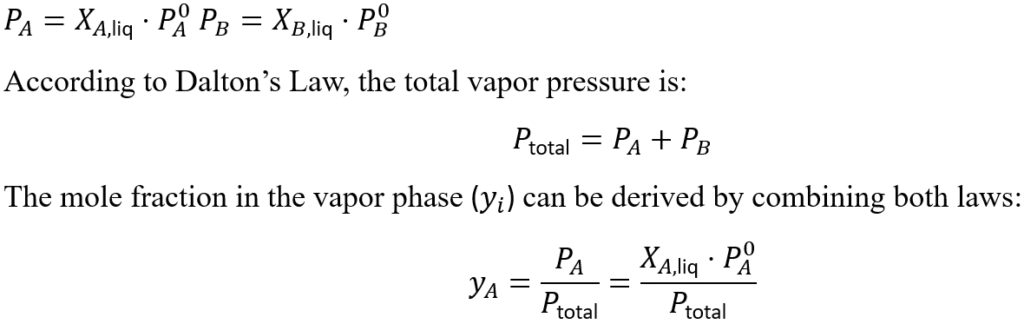
This relationship is critical in processes like distillation, where the vapor composition determines separation efficiency.
Visualizing the Laws: Graphs and Data
To illustrate, let’s consider a solution of benzene (vapor pressure 100 mmHg at 25°C) and toluene (vapor pressure 30 mmHg at 25°C). Using Raoult’s Law, we can plot the partial and total vapor pressures against the mole fraction of benzene.
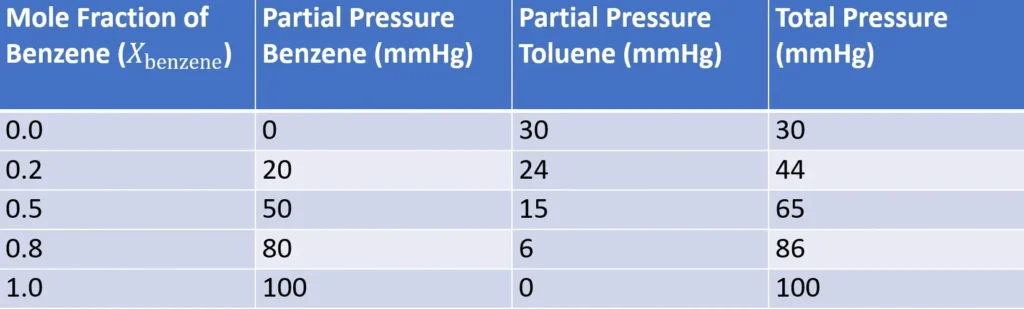
This graph shows the linear dependence of vapor pressure on mole fraction, a hallmark of ideal solutions. Dalton’s Law then sums these pressures to give the total vapor pressure.
Why These Laws Matter in Process Safety
In process industries, understanding Raoult’s and Dalton’s Laws is crucial for safety. Misjudging vapor pressures in a chemical reactor can lead to over-pressurization, risking explosions. Similarly, incorrect gas mixture calculations can cause unsafe conditions in storage tanks. For example, a 2010 incident at a refinery highlighted the importance of accurate vapor pressure predictions to prevent volatile emissions during distillation.
People Also Asked Questions with Answers
Can Raoult’s Law be used for non-ideal solutions?
Yes, but for non-ideal solutions, Raoult’s Law requires modifications using activity coefficients to account for deviations caused by differing intermolecular forces.
Why is Dalton’s Law important for air quality?
Dalton’s Law allows to calculate the partial pressures of gases like oxygen or different pollutants in the atmosphere for quality monitoring and emission control.
What is the difference between Raoult’s law and Henry’s law?
Henry’s Law says that the more pressure you put on a gas over a liquid, the more of that gas will dissolve in the liquid. Raoult’s Law, on the other hand, says that in a perfect liquid mixture, each part of the mix contributes to the total pressure based on how much of it is there and how easily it turns into a gas.
References:
- Atkins, P., & de Paula, J. (2014). Physical Chemistry. Oxford University Press.
- McCabe, W. L., Smith, J. C., & Harriott, P. (2005). Unit Operations of Chemical Engineering. McGraw-Hill.
- Levine, I. N. (2009). Physical Chemistry. McGraw-Hill.
- Smith, J. M., Van Ness, H. C., & Abbott, M. M. (2005). Introduction to Chemical Engineering Thermodynamics. McGraw-Hill.
- Seader, J. D., & Henley, E. J. (2006). Separation Process Principles. Wiley.
- https://www.britannica.com
- https://www.carolina.com