Hydrotreating, Hydroprocessing, or Hydrodesulfurization is a process in which the petroleum feed stream reacts with Hydrogen at high temperature and pressure in the presence of a catalyst, to remove the impurities Sulfur, Nitrogen, Oxygen, metals, chloride, etc. In the mid-1950s, the first naphtha catalytic hydrodesulfurization was commercialized to produce clean feed for the platforming plant. After that, numerous proprietary catalytic hydrodesulfurization processes, have developed. Currently, almost all petroleum oil refineries have one or more hydrotreating unit
Purpose of Hydrotreating Process
The purpose of the Hydrotreament process in an oil refinery is;
- To treat the final petroleum product like Kerosene, diesel, or naphtha
- To prepare feed for the next unit like naphtha for the platforming unit
- To produce an ultra-low sulfur product to meet environmental standards.
There are different types of hydrotreaters depending on the required product specifications and the type of product handled and applied to a wide range of petroleum streams from naphtha to reduced crude. The basic process of all the Hydrotreating units is the same. The following are some common hydrotreaters used in refineries:
- The naphtha hydrotreater treats naphtha before sending it to the reformer and isomerization unit.
- Kerosene hydrotreater removes sulfur and improves the smoke point in Kerosene.
- Diesel hydrotreater removes sulfur and nitrogen.
- The gas Oil hydrotreater treats gas oil before sending it to the fluid catalytic cracker unit (FCCU).
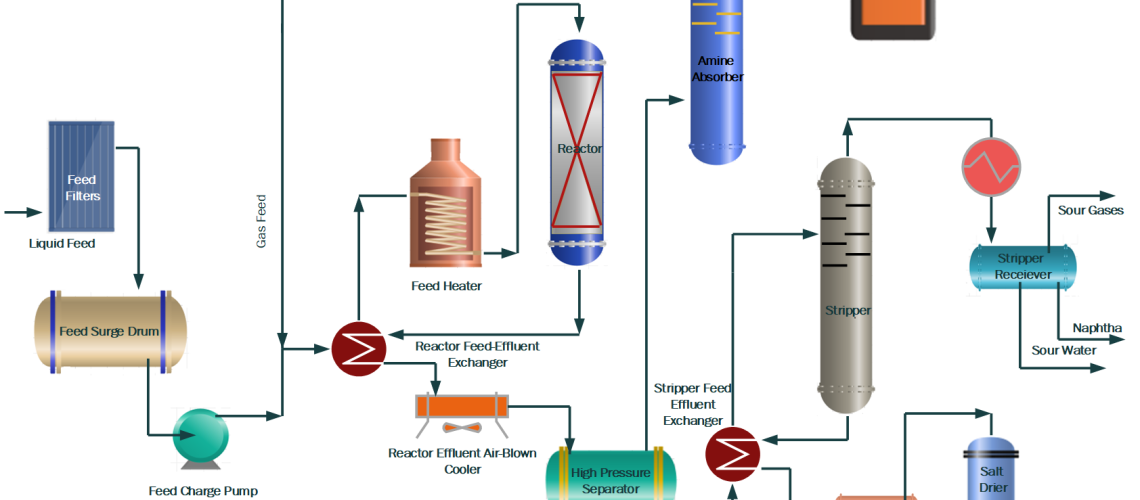
The process flow diagram of a typical Hydroprocessing unit is shown below.
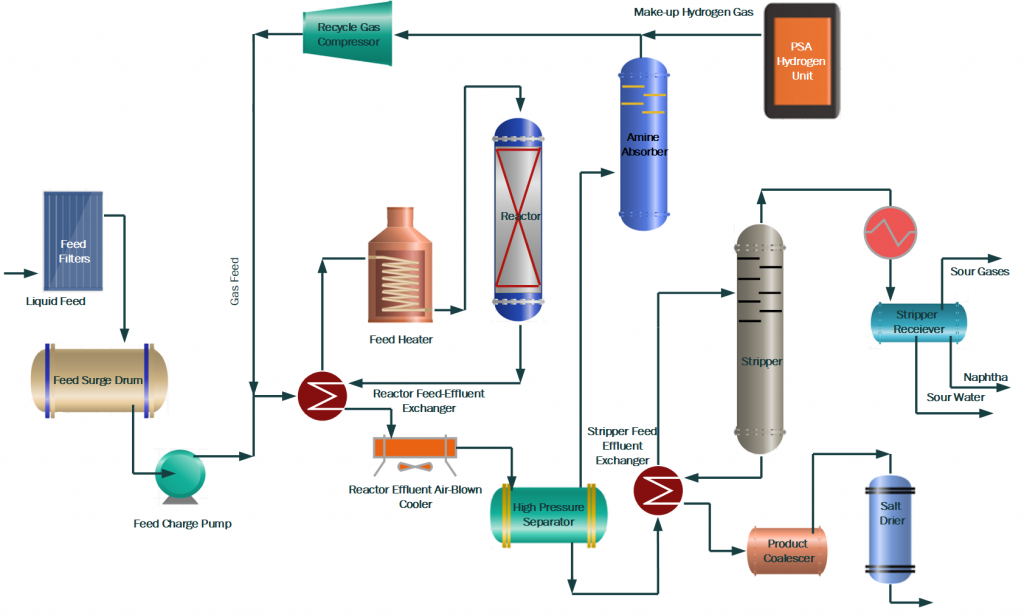
Following is the process description of the Hydrotreament plant which consists of different sections.
Feed Section
Feed Section receives the liquid feed from the upstream unit. Particles and moisture are removed before going to the feed storage tank. Major equipment includes a feed filtration system, feed storage tank, and feed pumps.
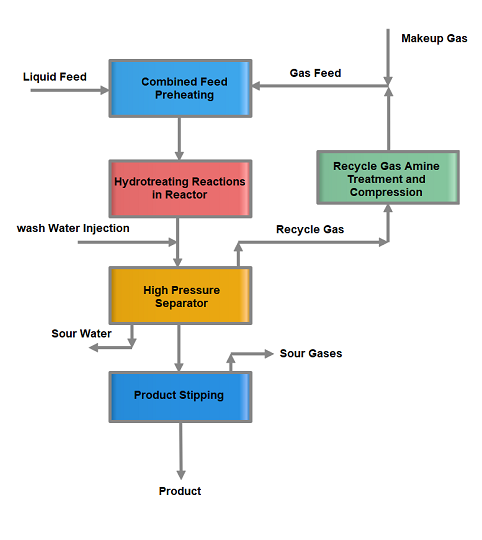
Feed Preheat Section heats the liquid and gas feed in a series of heat exchangers called feed heat exchangers. Combined feed is heated up by the reactor effluent fluid in these exchangers. The pre-heated feed is passed through a furnace and heated up as per reaction conditions for the hydrotreating reactions.
Reactor Section
In the Reactor Section Hydrotreating, reactions are carried out in a hydrotreating reactor at conditions of 300~400 °C temperature and 5~50 bar pressure. Pressure remains to fix while the reactor temperature is altered according to the product quality requirement. Temperature rise results in low Sulfur and Nitrogen in the product. In addition, a reduction in the feed endpoint and an increase in hydrogen purity also help in the increase in reactor activity to reduce impurities in the product.
A hydrotreating reactor is a fixed bed and downward flow reactor with Alumina base Ni-Mo (for Nitrogen removal and aromatics saturation) and Co-Mo (for sulfur removal and Olefin Saturation) hydrotreating catalysts. In addition, guard or grading catalysts are present at the top of the main catalyst to remove metals or particles from the reactor feed.
Major hydrotreating reactions are Desulfurization, Denitrogenation, Demetallation, Aromatics saturation, Olefin saturation, Oxygen removal, and chloride removal. All the Hydrotreating reactions are exothermic, resulting in a temperature rise across the hydrotreating reactor. For multi-bed reactors, bed temperatures are equally maintained by using inter-bed quench gas.
In the hydrotreating chemical reactions, organic Sulfur and Nitrogen are converted to inorganic forms H2S (hydrogen Sulfide) and NH3 (Ammonia). Metals are retained by guard or grading catalysts by hydro-demetallation reactions. Oxygen is converted to water (H2O). Chlorides are converted to HCL and are removed in the form of Ammonium Chloride salts at low temperatures.
The reactor effluent is cooled down in the feed exchangers by heating up the combined feed. Then wash water is injected into the reactor effluent to dissolve the salts of Ammonia, H2S, and Chlorides. This is the first stage of Sulfur and Nitrogen removal in the form of Ammonium salts. Wash water not only removes the salts but also helps to avoid fouling of exchangers due to salts deposition at low temperatures.
Further, the reactor effluent is sent to a separator where excess hydrogen gas along with H2S, sour water, and Hydrocarbon liquid are separated and sent to downstream sections for further processing.
Gas Treatment Section
In the Gas Treatment Section, separated gas also called recycle gas is treated with Lean Amine in a Scrubber to remove H2S. Rich amine is treated in the Amine treatment section to regenerate and reuse for scrubbing.
Treated gas is then combined with fresh makeup gas and then injected into the reactor section along with liquid feed by a recycle gas compressor. High purity of Hydrogen is required to prevent coke formation on the catalyst and to achieve desired reactions at low temperatures. A small amount of gas is also purged from the system to maintain the purity of the recycle gas.
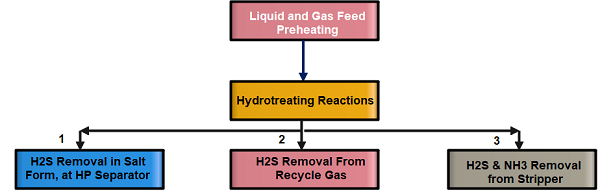
There are three stages of H2S and Ammonia removal 1. By wash water injection in the form of Sour Water 2. By Amine treatment for Recycle Gas 3. Along the lighter ends in the product Stripper.
Fractionation Section
In this section, hydrocarbon feed from the separator is heated using reactor effluent and stripper product fluid. Then this feed is sent to Stripper to remove H2S, NH3, and lighter hydrocarbon gases. Live steam or reboiler is used to strip out lighter elements from the stripper feed. Stripper off-gases are condensed and collected in a receiver. Hydrocarbon liquid is used as reflux, sour water collected in the boot of the vessel is transferred to the sour water section, and gases are routed to the refinery sour gases circuit for further treatment.
Stripped product is cooled down and treated to remove moisture in the product coalescer and salt drier. In addition, different chemicals can also be added to meet product specifications.
Hydrotreating Vs Hydrocracking
1. Hydrocracking is the process of converting high molecular weight Hydrocarbon items (VGO, Vacuum/atmospheric residues, etc.) into more valuable lighter petroleum products like LPG, gasoline, Kerosene, and diesel. Hydrotreating is the process of removing impurities like Sulfur, Nitrogen, Oxygen, metals, chloride, etc. from petroleum products like gasoline, kerosene, and diesel.
2. Hydrocracking is used for the conversion of HC into products while hydrotreating is used for feeds or final product treatment.
3. Hydrocracking requires severe operating conditions while hydrotreating uses mild conditions.
4. Hydrocracking uses both the treating and cracking functions of petroleum feed while hydrotreating uses only the treating of hydrocarbon.
For further information, discussion and queries please comment in the box below or contact admin@, or follow us on Facebook & LinkedIn.