The feedstock of hydroprocessing units contain a sufficient amount of Sulfur and Nitrogen. These Sulfur and Nitrogen are converted to hydrogen sulfide and ammonia in the reactor. As the reactor effluent material cools down there is a characteristic temperature at which these two compounds will sublimate as ammonium bisulfide (NH4HS), and ammonium chloride (NH4Cl) salts. If these salts are not removed, will foul and corrode the heat exchanger’s surface and result in a loss of heat transfer and corrosion.
The salts ammonium bisulfide (NH4HS) and ammonium chloride (NH4Cl) are both water-soluble and can easily be removed by injection of a sufficient amount of water. Wash water is injected to minimize ammonium salt fouling before Reactor Effluent Air Cooler (REAC) and before the last Reactor Feed/Reactor Effluent Exchanger. The wash water injection point is usually located upstream of the predicted location where salts will deposit. Corrosion by these aqueous salt solutions can occur if the wash water injection system is not correctly designed and operated.
Effects of Poor Wash Water System in Hydroprocessing Units
- Plugging of exchangers due to NH4HS & NH4Cl salts. These may include Reactor Effluent Air Cooled Exchanger and Reactor Effluent-Stripper Feed shell and tube heat exchanger.
- If NH4HS & NH4Cl salts are not removed through water washing this material will foul the heat exchanger surface and result in a loss of heat transfer. If left uncorrected, plugging of exchanger tubes can eventually occur.
- If the wash water injection is stopped for a longer period of time (depending upon unit design), the H2S and NH3 may accumulate in the recycle gas and result in a sudden loss in catalyst activity. The activity can resume to normal once wash water is re-established.
- Aqueous NH4HS forms an alkaline, sour water solution. These solutions can become a significant corrosion concern with increasing concentration, increasing partial pressure of H2S, and increasing velocity and turbulence.
- Solid NH4Cl salt can foul equipment and plug heat exchanger tubes. If sufficient water is not present to dissolve and dilute the salt deposits, any wet deposits or concentrated salt solutions will be highly corrosive.
- A low flow of wash water may result in a high concentration of Ammonium Salts in a High-Pressure Separator and hence highly corrosive sour water.
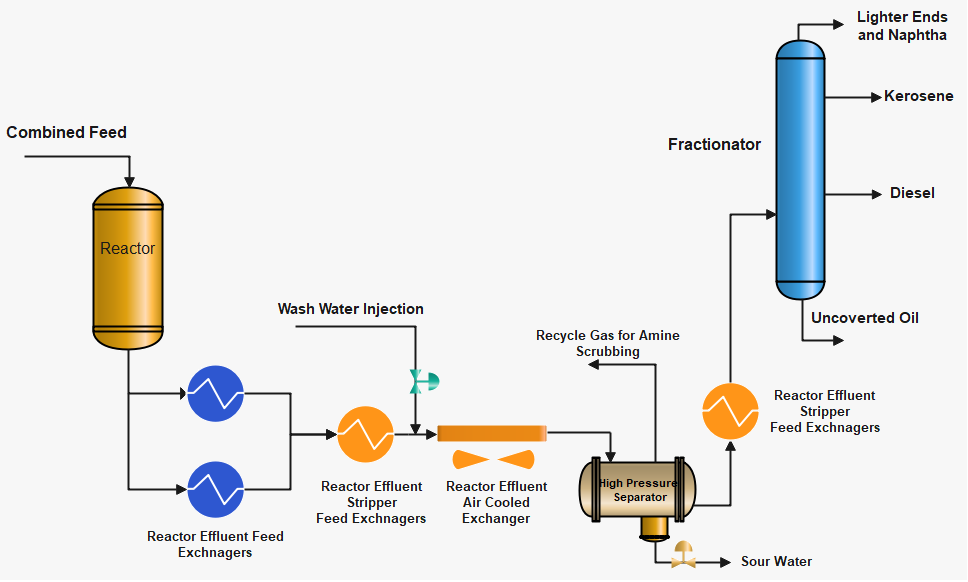
Formation of NH4HS and NH4Cl Salts
As the reactor effluent cools down the H2S, NH3 and HCl will sublimate to NH4HS and NH4Cl at characteristic temperatures and deposit on piping and equipment. The point at which this occurs is a function of the product of the partial pressures of each compound in the reactor effluent material. Figures A & B below show this relationship for ammonium bisulfide and ammonium chloride, respectively. In most cases, ammonium bisulfide will sublimate at temperatures most likely to occur in the air- or water-cooled effluent exchanger. But NH4Cl can sublimate in reactor effluent exchangers just before the air-cooled exchanger as NH4Cl can deposit above 200 °C.
A factor that is called Kp, which is the product of the mol% concentrations of ammonia and hydrogen sulfide, appeared to correlate with the corrosivity of the process fluid. Higher Kp values tended to lead to more corrosion. Similarly, the higher the mole % concentration of Chlorides and H2S higher will be the chances of NH4Cl formation and the higher will be the corrosion or equipment plugging.
Corrosion can be caused by the deposition of either ammonium chlorides or ammonium bisulfides or both. The deposition temperatures of the two salts are considerably different. Ammonium chloride usually deposits at 176~204 °C (350°F – 400°F), whereas bisulfides deposit in the range of 26~65 °C (80°F – 150°F). Deposition temperatures of Ammonim Bisulfide and Ammonium Chloride increase with the amount of chloride or nitrogen in the feed.

Calculating Deposition Temperature of NH4Cl (Ammonium Bisulfide)
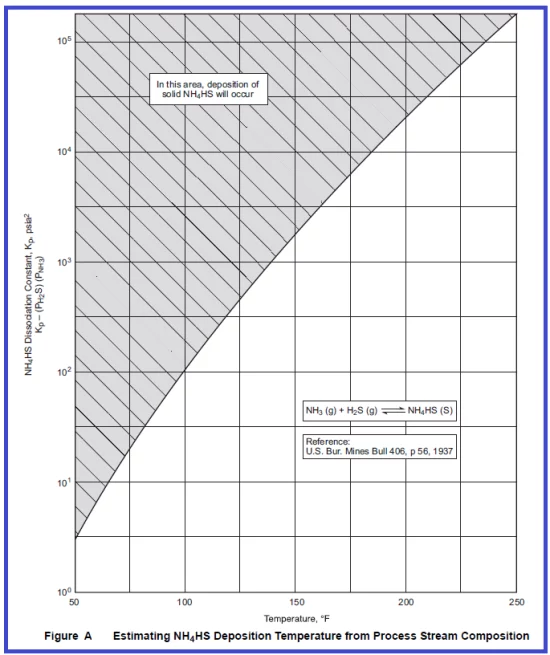
By using Figures A &B given above deposition temperature of NH4HS & NH4Cl can be calculated; First, calculate the Kp value as follows;
Kp= Partial Pressure of Ammonia* Partial Pressure of H2S
Partial Pressure of Ammnia=(mole of NH3 in Vapour Phase/total moles of Vapour Phase)*Total System Pressure
Partial Pressure of H2S= (mole of H2S in vapour phase/total moles in vapour phase)*Total System Pressure
Mole fractions of H2S and NH3 can be calculated using design or simulation data of the unit.
For example, Ammonia moles in the vapour phase are 5 Kg mole/hr, H2S 70 Kg mole/hr, total 1700 Kg mole vapour flow, System Pressure= 569 psi (a)
Partial pressure of NH3= (5/1700)*569= 1.67 psi & Partial Pressure of H2S: 70/1700=0.041*569=23.4 psi
Kp=1.67*23.4=39 (psi*psi)
The estimated Deposition Temperature of NH4HS obtained from Figure “A” using kp value 39 is ~ 80 °F. Similarly, if the Kp value is 100 then the deposition temperature of NH4HS would be 100 °F.
Calculating NH4Cl Deposition Temperature
The same procedure can be used to estimate the NH4Cl deposition temperature from Figure B above.
Partial pressure of NH3= (5/1700)*569= 1.67 psi, Partial Pressure of HCl= 0.2/1700*569=0.067
Kp= 1.67*0.67= 0.112 psi2 = 11.2*10-2
So NH4Cl the deposition temperature would be ~410 °F ( 210 °C).
Design and Operating Guidelines for Wash Water Injection
1. Wash Water Failure
The first corrective action is to fix the water injection as soon as possible. For a short duration partial wash water loss, reactor temperature should be lower as wash water is reduced. The unit load can also be reduced to reduce salts formation. Further, feed streams containing high concentrations of Nitrogen should be removed if possible to reduce the formation of Ammonia or Ammonium salt. Wash water should be reintroduced as soon as possible. Without wash water, the unit will need to be shut down. Moreover, the hydrotreating units with low feed Sulfur and Nitrogen can continue their operation without wash water for up to 8 hours. But the wash water failure time depends upon the designer’s guidelines.
2. Quality of Wash Water
The primary sources of wash water are stripped sour water, steam condensate, and boiler feedwater. Control good wash water quality by using condensate and minimizing stripper sour water reuse.
- Cyanides: If stripped sour water is used to supplement fresh water then cyanides can be a concern which can cause hydrogen blistering, phenols, which can cause foaming in the high-pressure separator. To avoid the buildup of dissolved and suspended solids, it is not recommended that stripped sour water alone be used as wash water. If necessary, limit stripped sour water to <50% of the total wash water as per requirement.
- Oxygen: The presence of oxygen increases the potential corrosion due to chloride pitting at and just downstream of the water injection point. Oxygen also increases corrosion due to sulfides, especially at the lower concentrations of bisulfide (lower pH) present at the water injection point. Oxygen is a strong oxidizer and will react with the bisulfide ion to form elemental sulfur. At certain lower pH and temperature conditions, the elemental sulfur is stable and will cause fouling and corrosion in this wet sour system. However, at pH conditions above about 8, the elemental sulfur will subsequently react with NH4HS in the sour water to form ammonium polysulfide, which can act as a corrosion inhibitor further downstream in this wet sour system.
- Iron levels should be low because iron in the water will form insoluble iron sulfide and could deposit in the tubes and equipment. For instance, 50 gpm (11.3 m3/h) of wash water containing only 1 ppm of iron will form over 300 lbs (14 kg) of iron sulfide in a year.
- Suspended solids should be kept to a minimum as well. Particulates can plug the spray nozzle or quill depending upon the design, regardless of alloy.

3. REAC Inlet and Outlet Piping
Balanced flow through the air cooler banks and inlet and outlet piping is important to reduce the potential for localized corrosion and fouling of the piping and air cooler tubes. An unbalanced flow can create high flow rates in some banks and low flow rates in others, and variations in the water distribution. These influence the type, amount, and location of corrosion and fouling.
Symmetrical piping to and from the air-cooled exchanger is the most desirable configuration. Nonsymmetrical piping can lead to unequal distribution of reactor effluent to each bundle resulting in localized high ammonium bisulfide or ammonium chloride concentrations and subsequent corrosion of the air cooler bundles and associated piping.

4. Quantity of Wash Water
Sufficient water should be injected to ensure at least 20% of the water remains liquid after injection. This is to avoid flashing all the water and creating an acidic environment when the first drop of water begins to condense and produce an acceptable NH4HS concentration in the separator water. The quantity of water to be injected is dictated by two criteria:
a) Producing an acceptable NH4HS concentration in the separator water,
b) Allowing sufficient free water to exist at the injection point (i.e. an aqueous phase exists downstream of the injection point).
5. Concentration of Ammonium Salts in Sour Water
Another measure of corrosivity is the concentration of ammonium bisulfide in the separator sour water. Sufficient water should be injected to maintain the concentration of ammonium bisulfide in the sour water produced in the boot of the High-Pressure Separator below 8 wt%. This will minimize the potential corrosivity of the sour water in the reactor effluent condenser tubes and outlet piping.
6. Wash Water Injection Rate
The minimum wash water injection rate is 5 vol% of the fresh feed. It depends upon the unit design conditions and can be as low as 3 %. Further, hydrotreating units with very low salt formation can also be designed with an intermittent injection of wash water. But keep in mind the NH4HS concentration in sour water produced should be within desired rate i.e. maximum of 8 %.
7. Velocity Through Tubes
The velocity through the tubes should be 3-6 m/sec. As the NH4HS concentration increases, regions of high velocity or turbulence pose a corrosion problem. Regions of stagnant or very low flow can be prone to fouling and under-deposit corrosion.
10. Chlorides in Feedstock
Chlorides entering the unit in both the hydrocarbon feed stream and the hydrogen stream are converted to HCl in the hydroprocessing reaction. HCl can promote deterioration in the unit through aqueous HCl corrosion, NH4Cl salt fouling, and corrosion by wet NH4Cl deposits.For units containing high chlorides, aqueous HCl corrosion can be aggressive. One location prone to corrosion is at the initial water condensation point, most often the wash water injection point.
Solid NH4Cl salt can form directly from the NH3 and HCl present in the reactor effluent stream, depending on their concentrations and temperatures. These salts usually deposit at temperatures above the water condensation point up to 400 °F (204 °C) or higher. The deposition is most likely in reactor effluent heat exchangers (upstream of the water injection), at the inlet end of the REAC, and in the top section and overhead system of the fractionator.
Top References
- Hydroprceossing for Clean Energy Frank Zhu, Richard Hoehn, Vasant Thakkar, Edwin Yuh
- A Study of Corrosion in Hydroprocessing Reactor Effluent Air Cooler Systems (API 932-A).
- Design, Materials, Fabrication, Operation, and Inspection Guidelines for Corrosion Control in Hydroprocessing Reactor Effluent Air Cooler (REAC) Systems (API 932-B).
For further information, discussion and queries please comment in the box below or contact us at admin@ or follow us on Facebook & LinkedIn.
Certified Functional Safety Professional (FSP, TÜV SÜD), Certified HAZOP & PHA Leader, LOPA Practitioner, and Specialist in SIL Verification & Functional Safety Lifecycle, with 18 years of professional experience in Plant Operations and Process Safety across Petroleum Refining and Fertilizer Complexes.
- Nasir Hussain
- Nasir Hussain
- Nasir Hussain
- Nasir Hussain
- Nasir Hussain
- Nasir Hussain






1 thought on “Wash Water Injection in Hydroprocessing Units”
Excellent work appreciated 👍