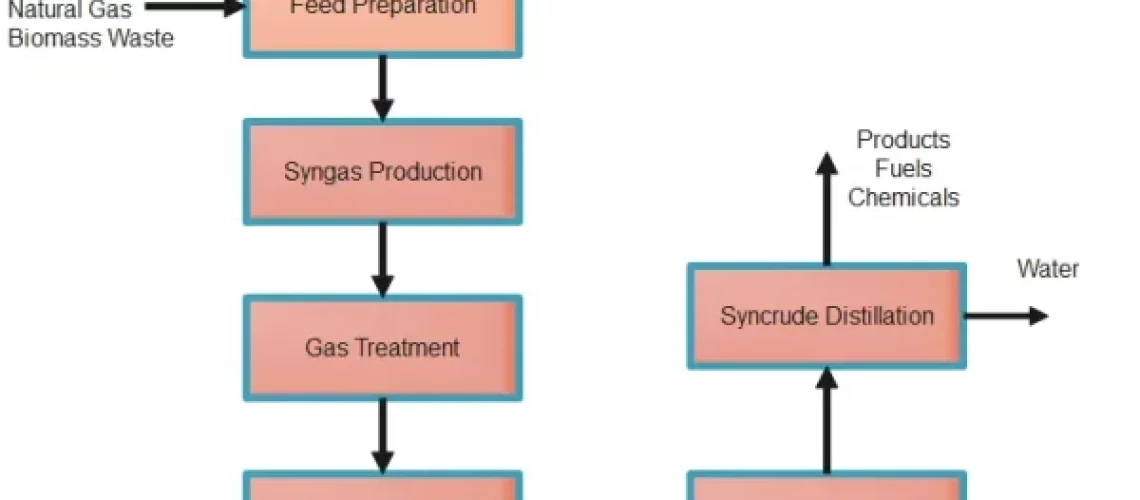
Liquid hydrocarbons like gasoline, diesel, and jet fuel can be produced from a mixture of carbon monoxide and hydrogen (syngas) via the Fischer-Tropsch process. In 1925, German scientists Franz Fischer and Hans Tropsch created the technique, which has subsequently found widespread use in a variety of commercial settings.
Using a catalyst, the Fischer-Tropsch (FT) process transforms syngas (a combination of carbon monoxide and hydrogen) into liquid hydrocarbons. The catalyst used in the Fischer-Tropsch process is typically based on iron, cobalt, or ruthenium metals.
Due to their low cost and high activity, iron-based catalysts are the most commonly used catalysts in the Fischer-Tropsch process. They are often prepared by impregnating an iron salt onto a support material, such as alumina or silica, and then reducing the iron salt with a reducing agent, such as hydrogen. The resulting iron nanoparticles serve as active sites for the reaction. The catalyst is periodically regenerated by removing accumulated impurities and reactivating the metallic nanoparticles.
Coal, biomass, and natural gas can all be gasified to produce carbon monoxide and hydrogen which is called Synthesis gas. The syngas is treated to remove impurities such as sulphur and other contaminants. The syngas mixture is fed into a fixed-bed reactor containing the activated catalyst. The syngas reacts on the surface of the catalyst to form liquid hydrocarbons, including diesel, gasoline, and jet fuel.

The liquid hydrocarbons are separated from the reaction products by distillation and other separation techniques. The liquid hydrocarbons are further upgraded through refining processes to produce final products, such as gasoline, diesel, and jet fuel.
Fischer-Tropsch Process Applications
The Fischer-Tropsch process offers great potential for lowering industrial greenhouse gas emissions and increasing the availability of alternative fuels.The Fischer-Tropsch process has a number of applications, including:
1. Fuel Production
The Fischer-Tropsch process is most often used to create liquid hydrocarbon fuels. It can be refined into numerous fuel types, such as gasoline, diesel, and jet fuel.
2. Chemical Production
Methanol and olefins are just two of the many compounds that can be made using the Fischer-Tropsch method.
3. Carbon Capture
Greenhouse gas emissions from manufacturing operations can be reduced by including carbon capture and storage (CCS) technologies in the process.
4. Waste-to-energy
Municipal solid waste and agricultural waste are two examples of waste materials that can be processed using the Fischer-Tropsch process to produce liquid hydrocarbons.
5. Military Applications
Producing fuel for tanks and other military equipment is just one of the many military uses for the Fischer-Tropsch process[3].
References
- [1] R. Xu, P. S. Vengsarkar, D. Roe, and C. B. Roberts, “Fischer-Tropsch Synthesis Performance of Supported Nano-Iron Catalysts Synthesized by a Gas-Expanded Liquid Deposition Technique”.
- [2] A. L. Garden and E. Skúlason, “The Mechanism of Industrial Ammonia Synthesis Revisited: Calculations of the Role of the Associative Mechanism,” Journal of Physical Chemistry C
- [3] J. Hu, F. Yu, and Y. Lu, “Application of fischer-tropsch synthesis in biomass to liquid conversion,” Catalysts, vol. 2.