Home › Forums › Petro Solutions Forum › Hydroprocessing › Calculating the Amount of DMDS Required to Complete the Sulfiding
Tagged: calculations for DMDS required for sulfiding, DMDS for Sulfiding, Hydrotreating Catalysts, stoichiometric calculations for Sulfiding, Sulfiding reactions
- This topic has 0 replies, 1 voice, and was last updated 4 years, 5 months ago by
 Nasir Hussain.
Nasir Hussain.
-
AuthorPosts
-
-
June 6, 2021 at 10:54 am #1516
 Nasir HussainKeymaster
Nasir HussainKeymasterThe required quantity of Sulfur depends upon the amount of metals present in the catalyst. The composition or amount of the catalyst metal to be sulfided is provided by the catalyst supplier. So first find the exact composition of the catalyst then calculate the stoichiometric amount of Sulfur needed for sulfiding by these reactions as given in the table below.
Details about the sulfiding procedure can be found at Sulfiding of Hydrotreating Catalysts and its TroubleshootingMolecular weights are of Tungsten 183.84, Molybdenum 95.95, Oxygen 15.99, Hydrogen 1.0, Nickel 58.69, Sulfur 32, and Cobalt 58.93.
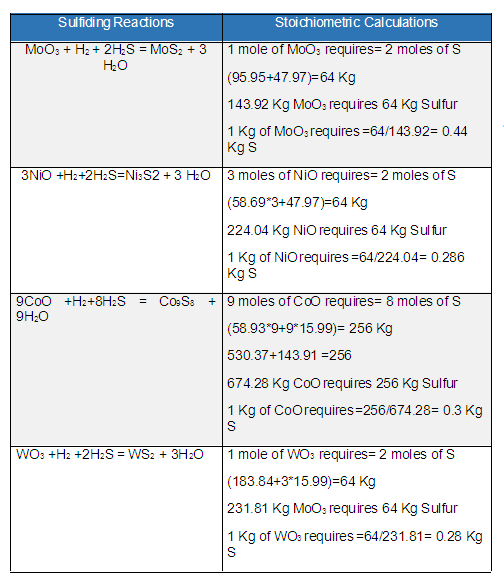
From the stoichiometric calculations as per the above image, the summary is as follows;
1 Kg of MoO3 requires 0.44 Kg of S
1 Kg of NiO requires 0.286 Kg of S
1 Kg of CoO requires 0.3 Kg of S
1 Kg of WO3 requires 0.28 Kg of SFor example, the hydrotreating catalyst having the composition 12 % Mo (12 Ton), 5 % (5 Ton) Ni, and 5 % Co (5 Ton), then 100 Ton of catalyst will require the sulfur using the unit method as follow;
1 Ton of MoO3 requires = 0.44 Ton of S
12 Tons of MoO3 requires = 12* 0.44= 5.28 Ton Sulfur
1 Ton of NiO requires = 0.286 Ton of S
5 Ton of NiO requires = 5*0.286 = 1.43 Ton
1 Ton of CoO requires = 0.3 Ton of S
5 Ton of CoO requires = 0.3*5 = 1.5 Ton of S
Total Sulfur required for Sulfiding = 5.28+1.43+1.5= 8.21 TonsTotal Sulfur required for sulfiding the 100 Ton of catalyst having composition 12 % Mo, 5 % Ni and 5 % Co is 8.21 Tons of Sulfur.
The amount of sulfiding agent DMDS (contains 68 % weight of Sulfur) can be found as follows;
0.68 Ton of Sulfur = 1 Ton of DMDS
1 Ton of Sulfur = 1/0.68 =1.47 Tons of DMDS
8.21 Ton of Sulfur = 8.21*1.47= 12 Tons of DMDSSo for Sulfiding 100 ton Hydrotreating catalyst with the composition stated above, the stoichiometric amount required is 12 tons of DMDS. But the actual amount required to ensure complete sulfiding would be more than 12 tons (15~17 Tons).
-
-
AuthorPosts
- You must be logged in to reply to this topic.