Catalytic reforming is the chemical process in oil refineries for converting the low-value heavy naphtha fraction of crude oil into high octane reformate, aromatics rich feed-stock and hydrogen. High octane reformate is a premium blending component for a refinery gasoline pool with a 30~50 % share. The aromatics-rich (benzene, toluene, and xylene) product serves as a vital petrochemical feedstock. The hydrogen product is utilized in other refinery hydroprocessing units.
Naphtha catalytic reforming process is the key process in oil refining to meet the demands of gasoline fuel specifications and hydrogen gas for hydrotreating and isomerization units. But one bottleneck of high aromatics content in gasoline may restrict the naphtha reforming process due to strict environmental regulations.
Catalytic Reforming Processes
Naphtha catalytic reforming processes are classified as continuous, cyclic, or semi-regenerative depending upon the frequency and mode of reforming catalyst regeneration.
1. Continuous Reforming Process is designed to continuously remove small quantities of reforming catalyst from a live reactor, transfer to a regeneration section, regenerate and return back to the reactor system. In the moving-bed design, all the reforming reactors are stacked on top of one another. The reactors have a common catalyst bed system that moves as a column of particles from top to bottom of the reactor section. The exhausted catalyst with coke on its surface is withdrawn from the last reactor and sent to the regeneration reactor, where the catalyst is regenerated on a continuous basis. The continuous reforming process has the advantage of operation at low pressures and high severity. Additional benefits include elimination of downtime for catalyst regeneration and steady production of high octane reformate and hydrogen of constant purity.
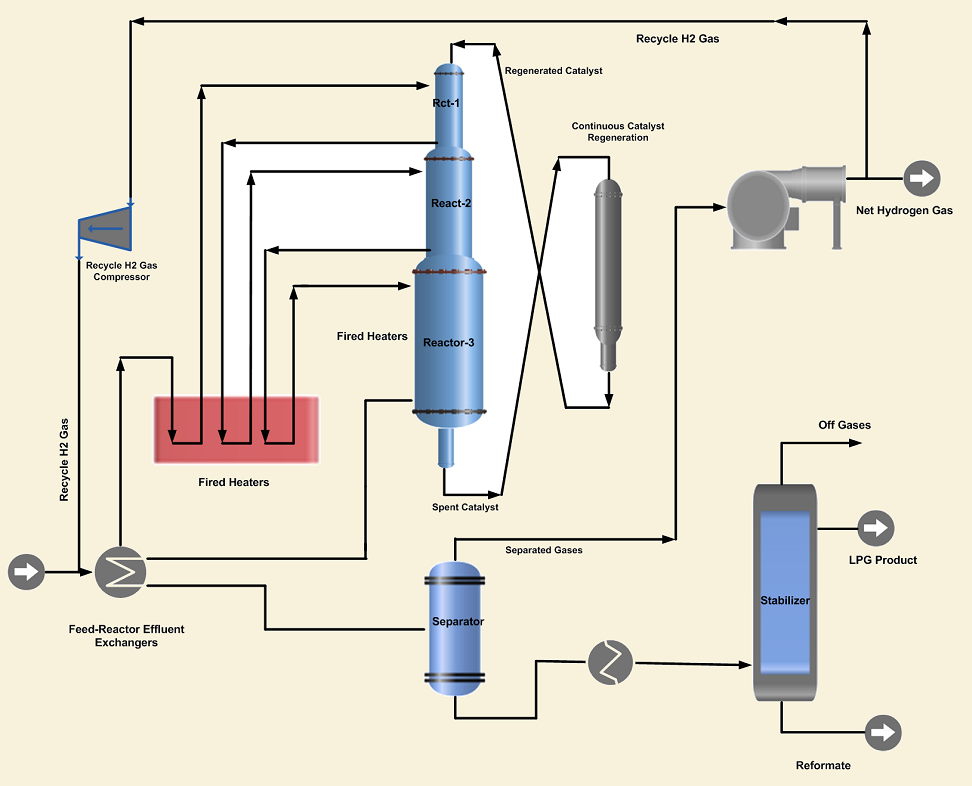
2. The Semi-regenerative Reforming Process on the other side requires the unit to be taken off-stream for catalyst regeneration. Depending upon the severity of the operation, regeneration is required at intervals of 1~2 years. High hydrogen recycles rates and operating pressures are utilized to minimize coke laydown and resultant loss of catalyst activity. It is a conventional reforming process, as the catalytic activity decreases the reformate RON and hydrogen purity drops. As the catalyst activity declines, reactor temperatures are increased to maintain the desired product quality.
3. The Cyclic Reforming Process is similar to the semi-regenerative process, having an additional swing reactor in which the catalyst can be regenerated without shutting down the unit. When the activity of the catalyst in any of the on-stream reactors drops below the desired level, then the swing reactor is taken into service and the exhausted reactor is isolated from the system. The catalyst in the isolated reactor is regenerated by utilizing hot air to burn off the carbon on the catalyst. After regeneration, it is used to replace the next reactor needing regeneration.
Description of Catalytic Reforming Process
The process description of all the naphtha reforming processes is almost the same except for the mode and frequency of catalyst regeneration.
The reforming unit comprises three sections: feedstock pretreatment section, reaction section, and product separation and stabilization section.
1. Feed Pre-treatment Section
All the refinery naphtha streams like straight run naphtha, cracked naphtha, coker naphtha, catalytic naphtha, etc. are treated and then get separated into light and heavy naphtha. Light naphtha is eliminated because it will crack to lighter products at reaction conditions and heavier is avoided because it promotes catalyst deactivation. The reformer feed is treated in a Naphtha hydrotreating unit to remove reforming catalysts poisons such as arsenic, lead, copper, sulfur, oxygen, and nitrogen compounds.
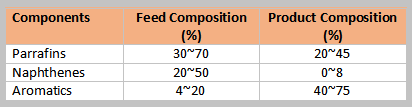
Typical composition of feed and products shown in the table below. Olefins present in the catalytic and thermal naphtha are removed in the hydrotreating of the reformer feed.
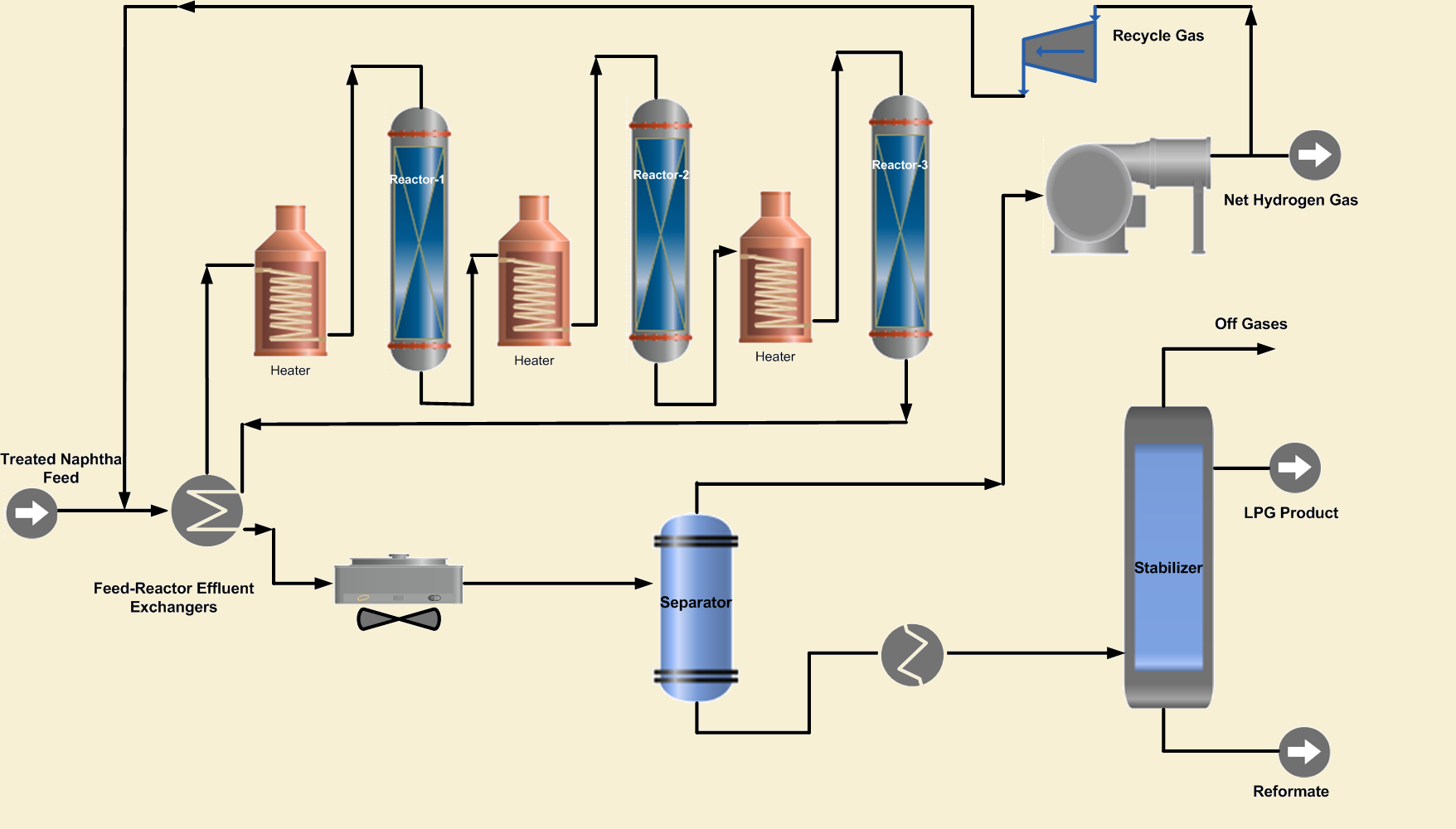
2. Reaction Section
The purpose of this section is to carry out the reactions of the catalytic reforming process to achieve the desired RON. This section of the conventional reformer consists of multibed reactors in series, with heaters between the reactors. Three or four reactors are frequently used to obtain a product with high octane number. The reactors contain the reforming catalyst that facilitates the desired reforming chemical reactions.
The hydrotreated naphtha feed is combined with hydrogen-rich recycle gas and preheated by heat exchange with the reactor effluent. Further the combined feed is heated in a fired heater before entering the first reactor. As the reforming reactions are endothermic that will cool the feed and product mix in the reactor. Therefore, the first reactor effluent is heated to the desired reaction temperature in a fired inter heater before entering the second reactor. In the same way, the effluent of the second reactor is heated and passed to the 3rd reactor to complete the desired reactions.
The temperature in the first reactor drops rapidly due to endothermic reactions such as dehydrogenation of cycloalkanes to aromatics. In the second reactor, endothermic reactions de-hydroisomerization of cyclopentane naphthenes take place at a lower rate than that of the first reactor.
In the last reforming reactor, reactions such as dehydrocyclization and cracking occur; however, slight variations in temperature are generally observed. The small change in the temperature is due to exothermic hydrocracking reactions in this reactor. Because some of the reactions are very fast, the first reactor is always smaller than the second, but the third and fourth are even larger.
3. Product Separation and Stabilization
The reactor effluent is cooled by exchanging heat with the combined feed and separated in a flash separator into gaseous and liquid product streams. The gaseous product consists of 60-90 mol % purity hydrogen and C1~ C4 lighter hydrocarbons.
Some portion of the hydrogen-rich gas is recycled and mixed with the naphtha feed. Its purpose is to suppress coke formation on the reactor’s catalysts. The net hydrogen-rich gas is sent to the refinery hydrogen system to be used in hydrotreating units or as a refinery fuel gas. The purity of net gas can be increased by recontacting the gas with the liquid reformate. This helps to increase the reformate yield along with rising in hydrogen purity.
The liquid product from the separator, which consists of C5~C10 hydrocarbons, is sent to the product stabilizer where lighter hydrocarbons C1~C4 plus LPG are removed from the high octane liquid product. The high octane reformate is sent to storage for blending with other naphtha streams to achieve the required RON blend.
Finally, the hydrogen and LPG products are further, treated to remove Chlorides from the product streams. Chloride removal is necessary, to save downstream units from corrosion.
Key Catalytic Reforming Reactions
- Naphthene dehydrogenation reactions are rapid, endothermic reactions that convert naphthenes to aromatics and hydrogen; are favored by high temperature and low pressure.
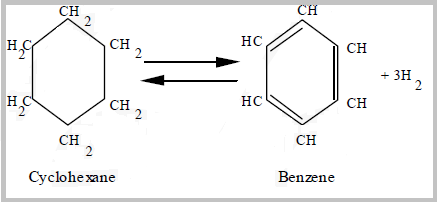
- The dehydrocyclization of paraffins is the most difficult reaction. It involves molecular rearrangement of paraffins to form a naphthene then subsequent aromatization of the naphthene yields a noticeable octane increase.
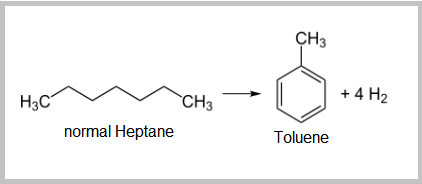
- The isomerization of paraffins is important to convert linear paraffins into higher-octane branched paraffins. These reactions are fast, slightly exothermic, and the number of carbon atoms does not change.
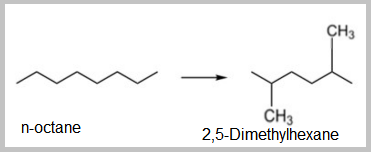
- The isomerization of Nephthenes like alkyl cyclopentane into alkyl cyclohexane involves a ring rearrangement and is desirable because dehydrogenation of the alkyl cyclohexane results in an aromatic.
- Some undesirable reactions also happen, like hydrocracking and alkylation of aromatics. Hydrocracking produces lighter products (hydrogen, LPG) and reduces reformate yield. Alkylation of aromatics produces heavier molecules that result in a high-end point of product and a high tendency of coke formation over the catalyst.
- Coke formation on the catalyst is an undesirable reaction that results from a very complex group of chemical reactions. The coke has a negative impact on catalyst performance as it covers the active surface area, and reduces its activity. Feedstock with high-endpoint, high reaction temperatures, low hydrogen to Hydrocarbon ratio, and low-pressure operation, increase the rate of coking over the catalyst.
Naphtha Reforming Catalyst
Typical catalytic reformer catalysts are bi-functional that comprised of platinum deposited on an alumina support, one or two metals added as promoters or modifiers, and chloride. The platinum on the alumina provides the metal function and the alumina along with chloride provides the acidic function. Both of the functions are required to achieve the desired reforming reactions.

Key Operating Parameters
Catalytic reformers are designed for flexibility in operation, whether for motor fuel production or to produce aromatics. To meet the varying demands of product quality, the reforming catalyst must respond to changes in unit operating conditions. The key operating variables which affect the performance of the catalyst and change the yield and quality of the reformate are feedstock properties, reaction temperature, space velocity, reaction pressure, and hydrogen-to-hydrocarbon mole ratio.
Naphthene Dehydrogenation and Parrafin dehydrocyclization are favored by high temperature and low pressure while hydrocracking, demethylation, and aromatic dealkylation require high pressure and high temperature. Further, the rate of isomerization of paraffin and naphthene increases with temperature.
For further discussions and comments please comment in the comment box below or contact at admin@thepeptrosolutions.com
Certified Functional Safety Professional (FSP, TÜV SÜD), Certified HAZOP & PHA Leader, LOPA Practitioner, and Specialist in SIL Verification & Functional Safety Lifecycle, with 18 years of professional experience in Plant Operations and Process Safety across Petroleum Refining and Fertilizer Complexes.
- Nasir Hussainhttps://thepetrosolutions.com/author/admin/
- Nasir Hussainhttps://thepetrosolutions.com/author/admin/
- Nasir Hussainhttps://thepetrosolutions.com/author/admin/
- Nasir Hussainhttps://thepetrosolutions.com/author/admin/







6 thoughts on “Catalytic Naphtha Reforming Process in Petroleum Refinery”
Nice explained and well written blog.
Great insights! I found your take on sustainable living incredibly practical.